Integrated Rate Laws
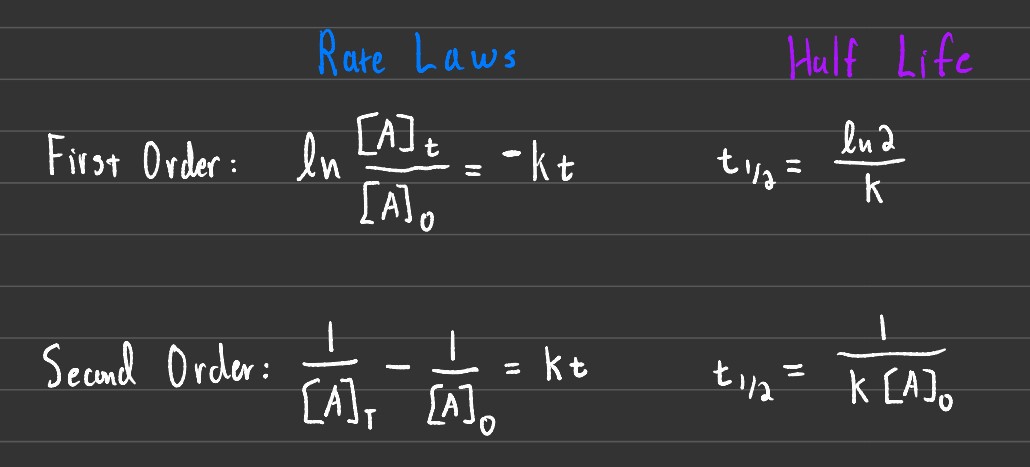
The image shown above is the generalized and concise version of both the rate laws and half-lives based on the order of the reaction. 1st order reactions are on the first row, while 2nd order reactions are on the second row.
Collision Theory
This theory has several parts which must all be true for a reaction to take place.
A collision has to occur for the reaction to take place.
Molecules must collide with enough energy.
- This ties directly to the activation energy.
Molecules must collide in the correct orientation.
Reaction Coordinate Diagrams
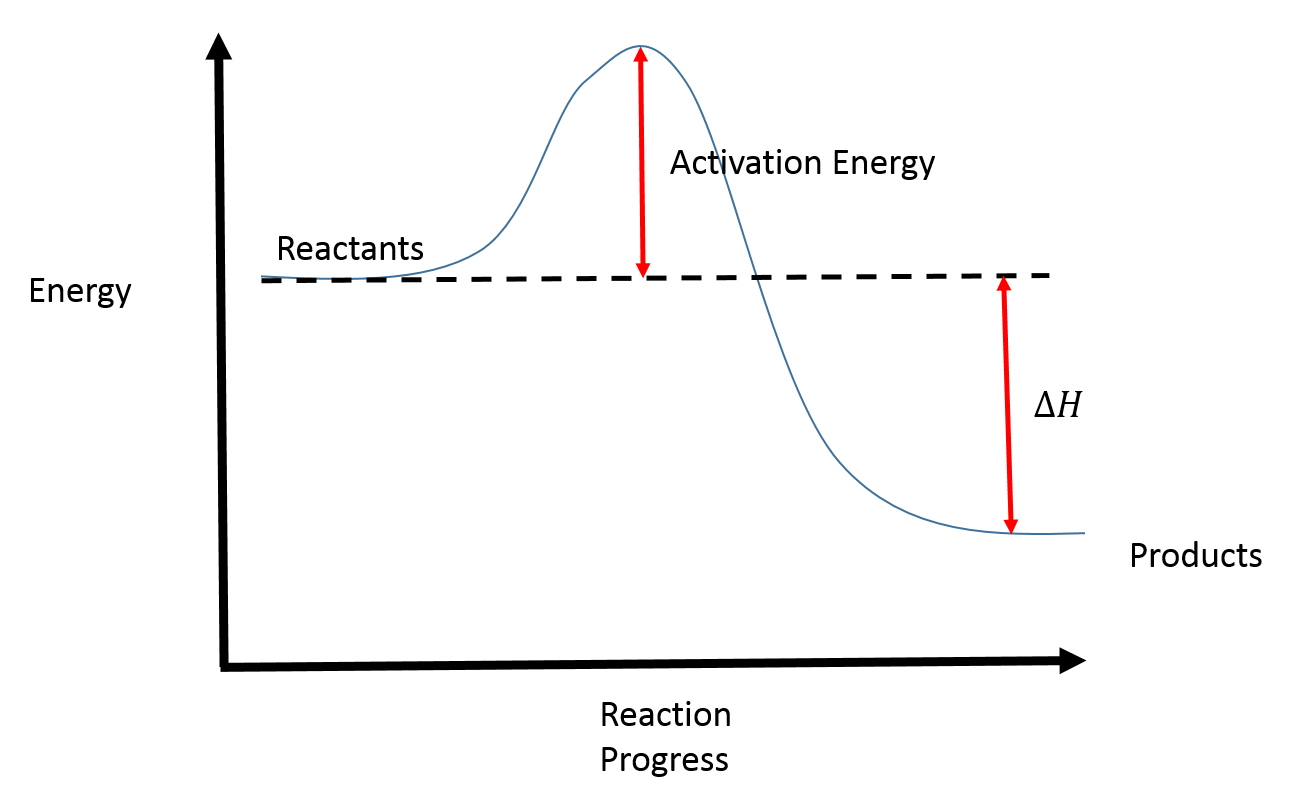
This diagram shows the process that a reaction goes through, including the activation energy required and shows the change in enthalpy (∆H). It's important to note that the activation energy is path dependent.
Arrhenius Equation
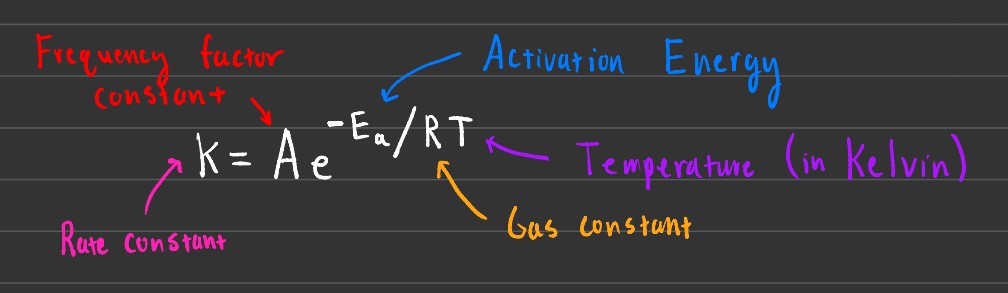
The purpose of this equation is to find the value of k, the rate constant. As temperature increases, so does the k constant (it increases exponentially).
Arepresents the frequency factor constant, which depends on the frequency of collisions along with the orientation of the molecules).Ris the gas constant, with a value of 8.314 J/mol K.
Note: The formula shown above is not the only way you will see/use it, there are several other ways you can manipulate the equation.
Reaction Mechanisms
Many, if not all, reactions occur in multiple elementary (or basic) steps. In some cases, reaction intermediates are produced and consumed by the reaction. What this means, is that sometimes a semi-product is created but then used to formulate the final product.
In general, the slowest step will determine the rate of a reaction. This slowest step usually occurs during the part with the largest activation energy.