Rate Laws

Rate laws essentially use concentration and relate it to the reaction rate.
The general formula is shown above, with several variables included.
k- This is a constant which depends on temperature, it is large for reactions with a fast rate. The units themselves vary depending on the reaction order.n, m- These 2 variables represent reaction orders, they have no correlation to the actual coefficients of the original equation. The overall reaction order is defined by n + m.a, b, c, d- These represent the actual coefficients of the original equation.A, B, C, D- These variables represent the elements or molecules that are used in the equation.
Reaction Order
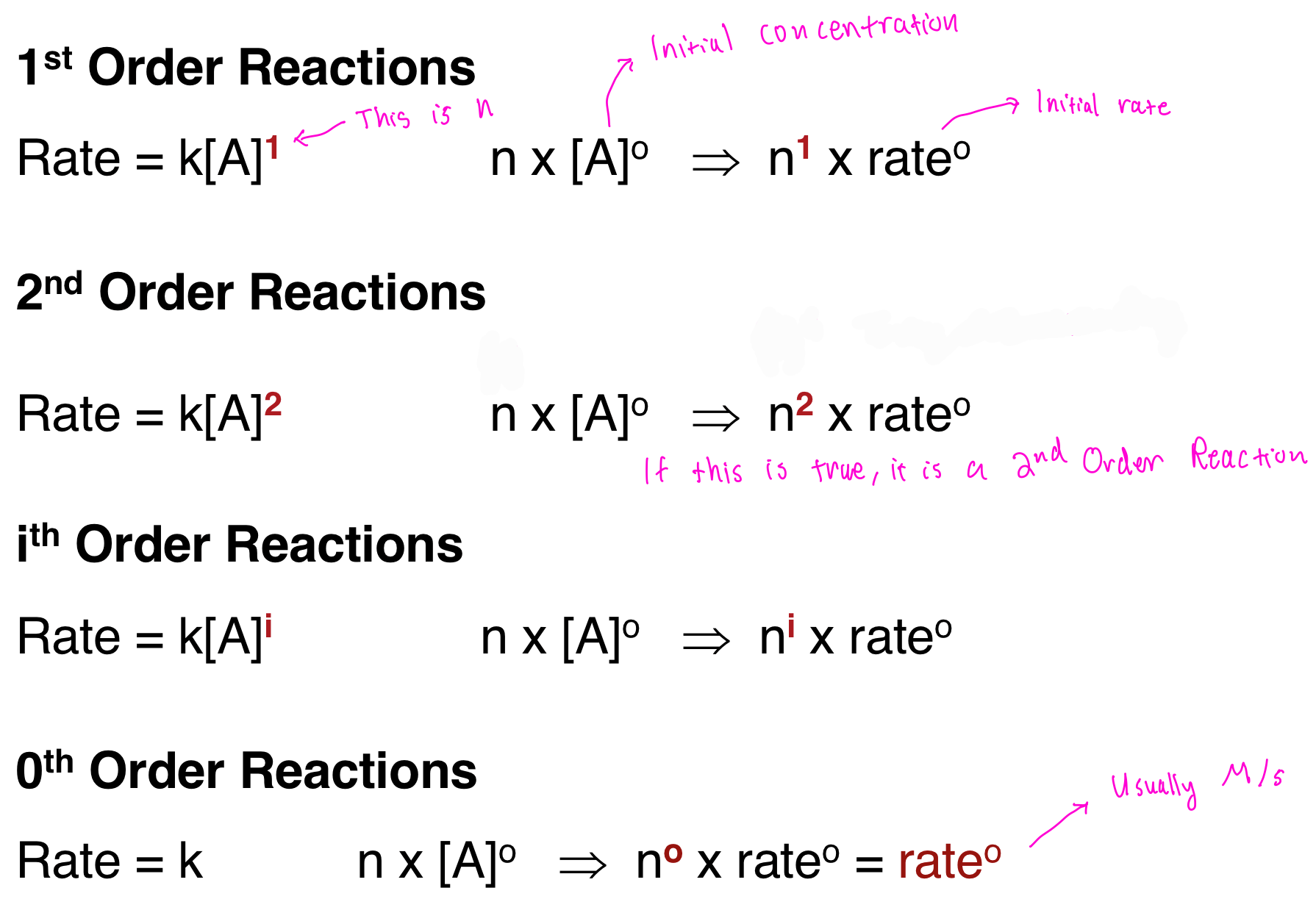
There are several orders of reaction, varying from 0th order up until the ith order. The most common you'll find are 0th order, 1st order, and 2nd order.
Each of these different ordered reactions relies on the value of n. The higher the order goes, the quicker the reaction takes place.
- 0th order reactions are linear in nature, they are constant and have a straight slope throughout.
Example
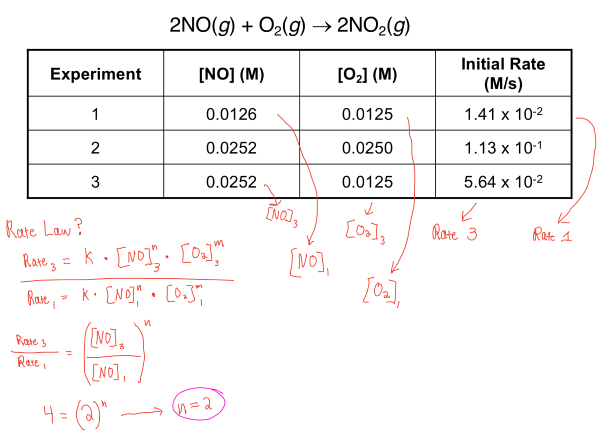
In the example above, you're asked to find the rate law of the equation. There are 2 parts to this because you need both n and m in order to find the overall reaction order.
So to do this you first have to find the ratio of 2 different rates, along with the ratio of 2 different molarities of 2 respective molecules/elements. In doing this you cancel out the k constant, and factor out the n, leaving you with a simplified equation. To find n value, you then solve by plugging in the given values and finding that n = 2.
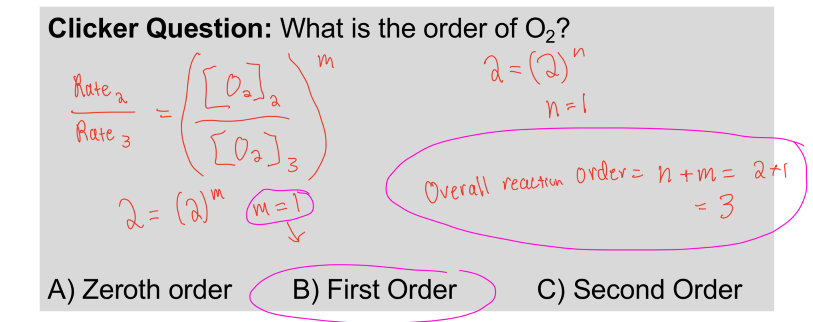
In the 2nd part of the question, you're asked to find the order of O2. To find this, you need to again go through the same process as above. The resulting answer is m = 1, which when combined with n gives the overall reaction order which is 3!
Note: The answer is B) First order, because it asks what the order of O2 was and not the overall reaction order.