Ideal Gas Law
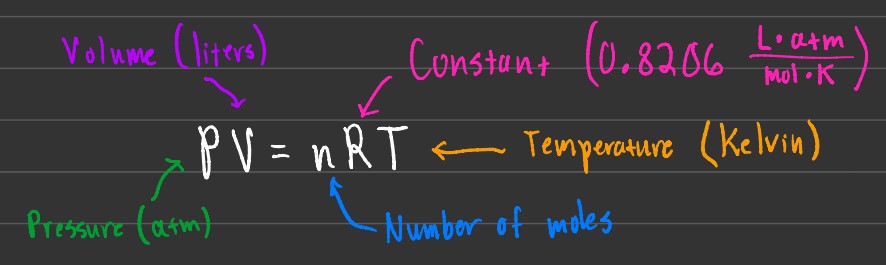
The Ideal Gas Law is the relation between pressure, volume, number of moles, and temperature. The R represents a constant, with the units shown above.
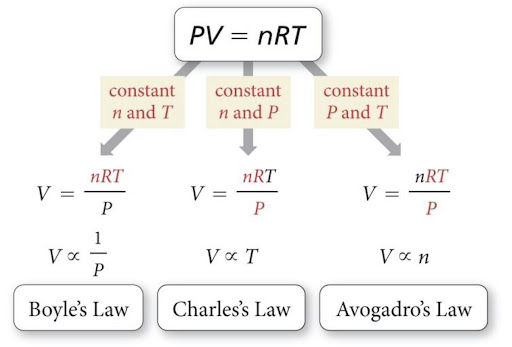
Boyle's Law states that volume (V) and pressure (P) are inversely proportional, so as one increases the other decreases and vice versa. This holds true when there is constant temperature and pressure.
Charles's Law states that volume (V) and temperature (T) are directly proportional, so as one increases so does the other. This is true whenever there is constant pressure and a constant number of moles of gas.
Avogadro's Law states that the number of moles of gas (n) and volume (V) are directly proportional, meaning that as one increases the other also increases. Note that temperature and pressure must be constant for this to be true.
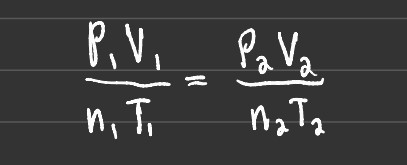
Additionally, you can solve for unknowns by setting two equations equal to each other (before and after). This is useful for finding the new pressure of a gas after the volume has decreased or another variable has changed.
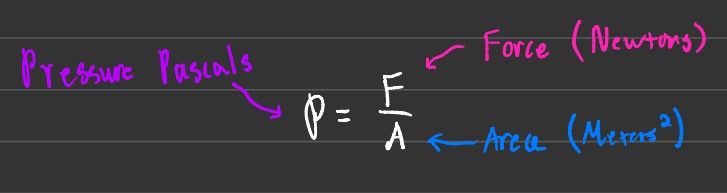
Another way to find pressure is to take the force in Newtons and divide it by the area in square meters.
Note: The result is pressure in pascals, which is different than what is used in the ideal gas equation. You must convert the units before utilizing the pressure.