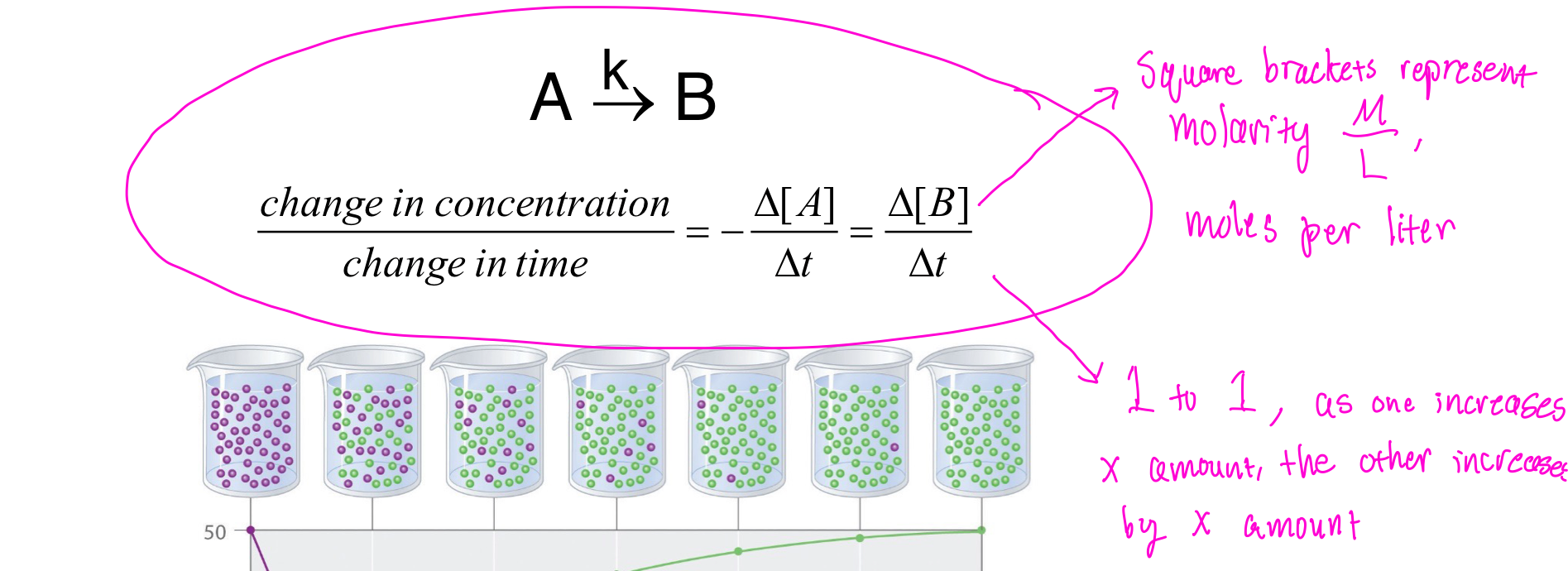
Reaction Rates
Reaction rates are very different depending on the substances involved, concentrations, temperatures, solvents, and surface area. Because of this, several formulas are used to keep track of the different reaction rates.
The main factor when it comes to reaction rates, however, is concentration. The unit of measurement is moles per liter or molarity as shown above. During reactions, the change in concentration over time is equal in both the reactants being used and the products being made. Different order reactions will be discussed in the next section.
It's important to note that these reactions are not linear, they take on more of a curve and are affected by what order reaction they are. In fact, the higher the concentration, the quicker the rate changes.
Example
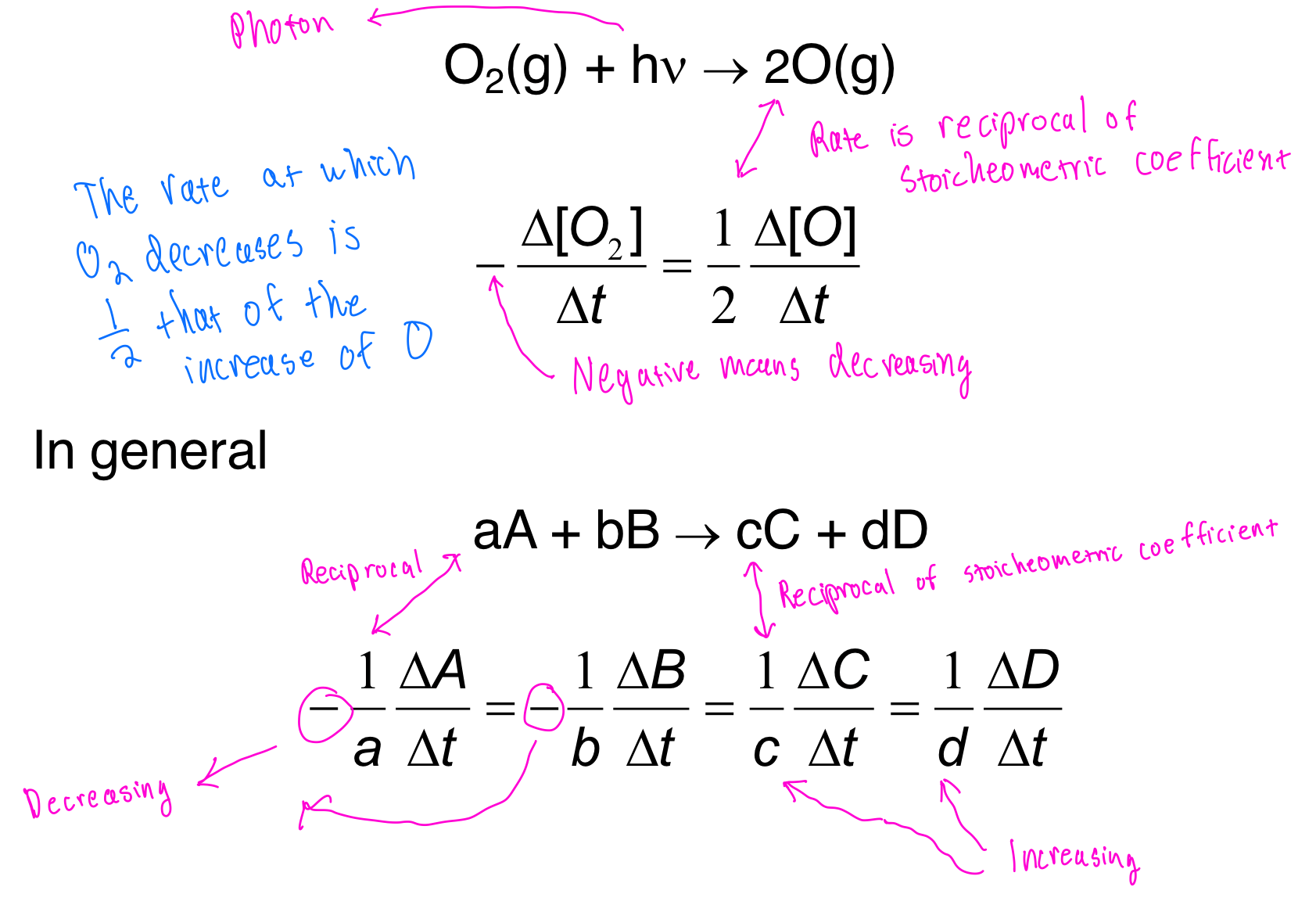
In the above example, O2 is being combined with a photon, producing 2O (g).
- Because O2 is being used up on the reactants side (decreasing), we make the coefficient for the rate negative.
- On the products side, the rate coefficient is 1/2 because you take the inverse of the original coefficient which was 2 (from 2O (g)).