Intermolecular Forces
Most of the forces that have been covered in chemistry have been within molecules, known more commonly as intramolecular forces. This section will cover intermolecular forces, known as the forces between molecules. This excludes covalent, ionic, and molecular bonds (they are all intramolecular).
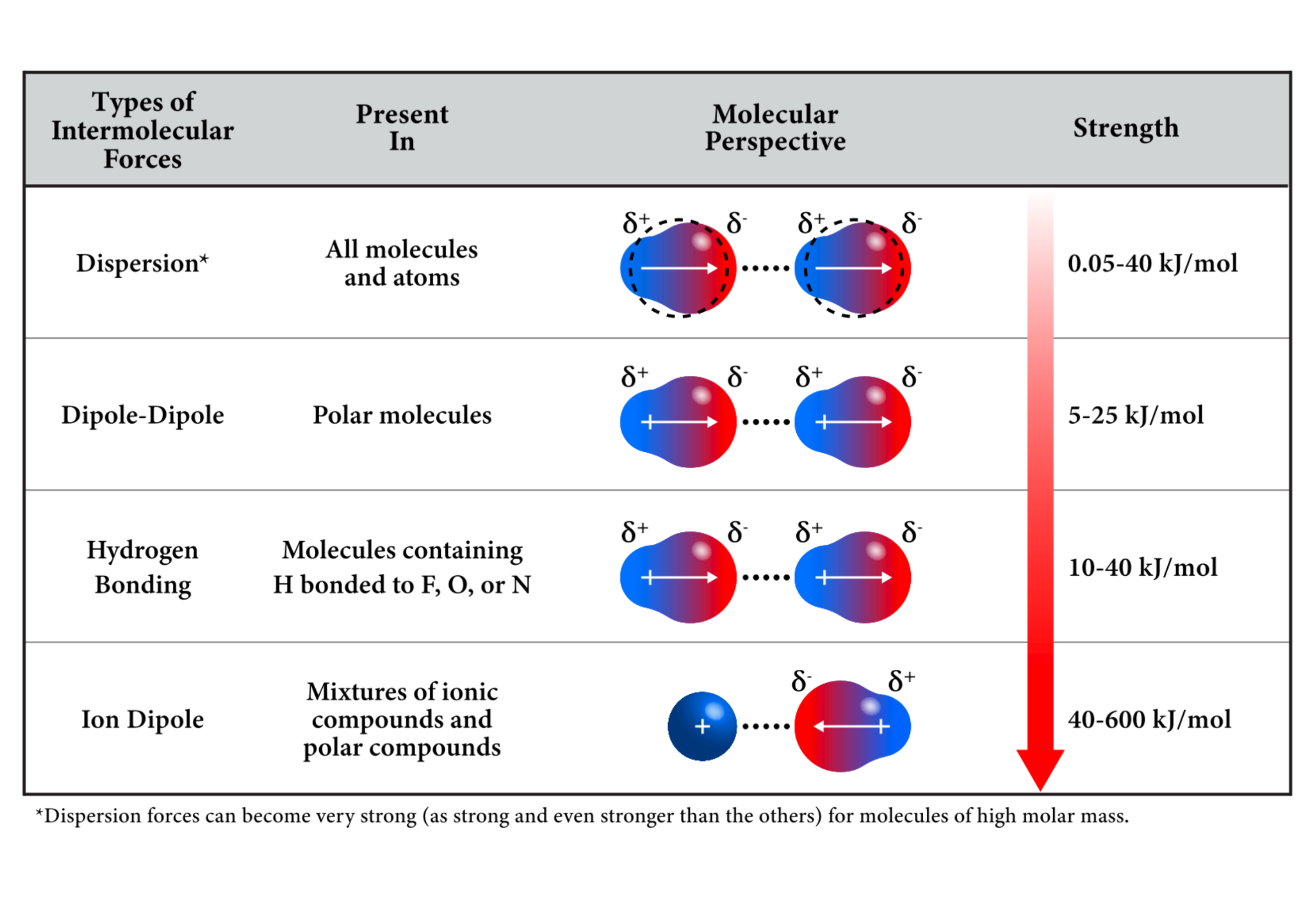
Types of Forces
There are several kinds of intermolecular forces, and it's important to note that these are significantly weaker than chemical bonds between atoms in a molecule.
Ion-Dipole (Strongest)
- These interactions occur between an ion and a polar molecule.
- When an ionic solid dissolves in a polar solvent, the interactions between the solvent molecule and the salt ions are called ion-dipole interactions.
Hydrogen Bonds
- These are dipole-dipole interactions that are extraordinarily strong when hydrogen is bonded to nitrogen, oxygen, or fluorine.
- This typically happens when hydrogen is bonded with a very electronegative element (as shown above by fluorine etc).
Dipole-dipole
- These kinds of interactions occur between 2 polar molecules.
- The more polar are molecule or substance is, the stronger the dipole-dipole forces are.
Dispersion Forces (Weakest)
- These are by far the weakest intermolecular forces.
- All molecules experience this force (except for noble gases).
- The strength of Dispersion Forces is directly related to the
polarizabilityof the molecule. - Generally, larger atoms are more polarizable and as such, they experience greater dispersion forces.