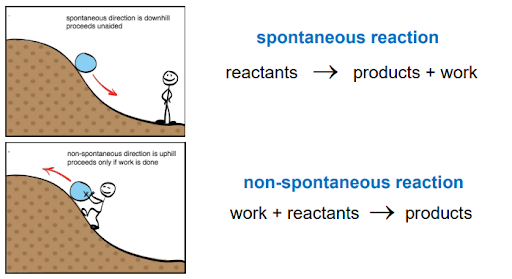
Spontaneity
Spontaneous processes occur without any ongoing outside intervention and are irreversible.
A process may be spontaneous at one temperature and non-spontaneous at another temperature. For example, imagine a glass of ice placed in a temperature-controlled room.
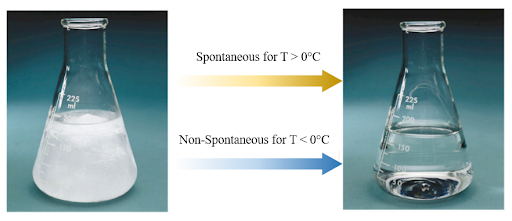
In the first scenario, the room has a temperature above 0°C (room temperature). In this room, the glass of ice will melt into liquid water (spontaneous process). In the second scenario, the room has a temperature below 0°C. In this, room the glass of ice stays as a glass of ice unless you purposely heat up the glass to melt the ice (a non-spontaneous process because you added work).
Spontaneous processes may be either endothermic (energy absorbed from surroundings) or exothermic (energy released into surroundings).