Limiting Reagants
Limiting reagent is the reactant that is completely consumed in a chemical reaction. Excess reagent is any reactant that remains or is left over after the limiting reactant has been completely consumed.
For example, take this chemical equation:
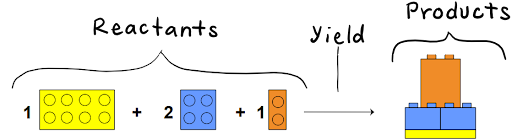
The image below represents all the reactants that we want to convert into products.
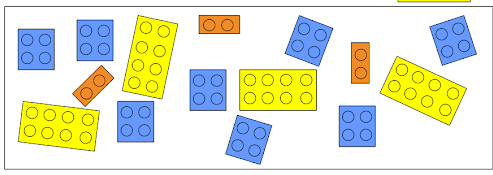
If we count the number of each reactant and divide it by the number needed to form one product we get that -
- Yellow cube can yield 4 products
- Blue cube can yield 4 products
- Orange cube can yield 3 products
Because the orange cube (reactant number 3) yields less than the others, we say that the orange cube is the limiting reactant of this chemical reaction.