Energy & Heat
Energy is the capacity to do work (denoted by w) or transfer heat (denoted by q). Usually, energy is measured in Joules or Calories.
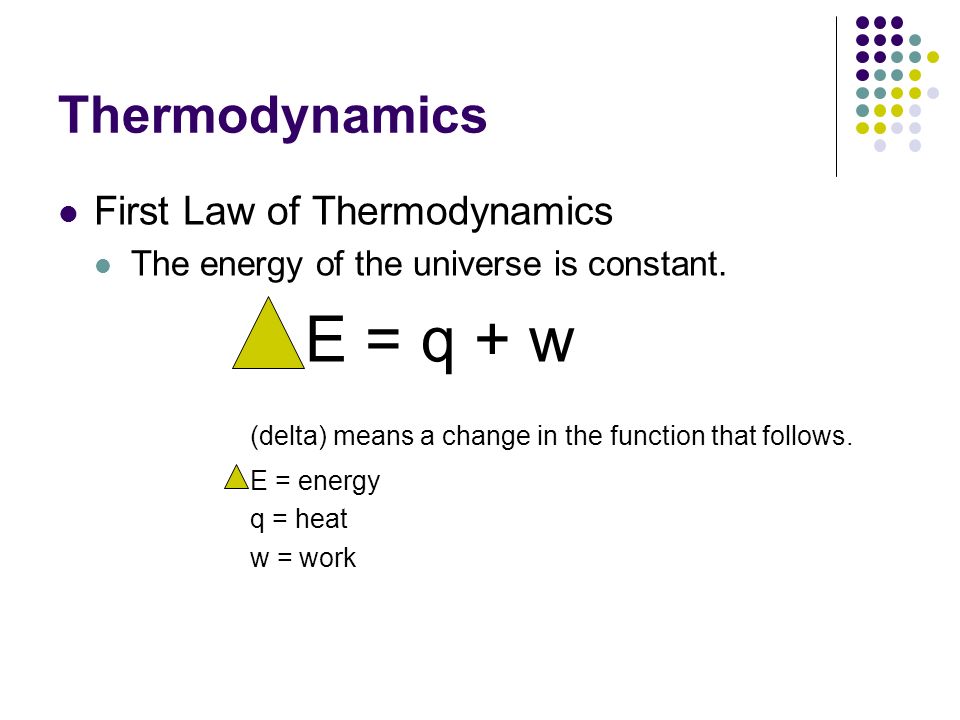
Before talking about what ∆E is (as shown above), it's important to understand what a system is and what the surroundings are.
- A system is the object being studied or the focus of the problem.
- The surroundings are everything else outside of this system/object.
∆E represents the change in energy, and the sign of this is very important.
- Whenever ∆E is negative, energy is lost to the surroundings.
- When ∆E is positive, however, energy is gained from the surroundings.
State & Path Functions
To describe a process, it is usually called a state or path function.
- State functions are dependent on the initial and final states of a system.
- Typically represented by uppercase variables.
- Ex. V, E, P, T
- Path functions on the other hand are dependent on the path that a system takes to get from the initial to the final state.
- They are represented by lowercase variables.
- Ex. q, w, t, d