Atoms
When looking at elements on the periodic table, there are several numbers adjacent to the symbol representing it. These include the atomic number, atomic symbol, and atomic weight. This sub-section will serve to help you understand what they mean and where they come from!
Base Units
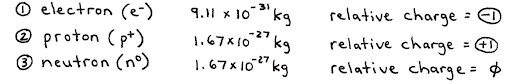
At the most fundamental level, atoms are comprised of 3 things:
- Protons
- Positively charged particles in the nucleus of an atom.
- Neutrons
- Neutral particles in the nucleus of the atom.
- Electrons
- Negatively charged particles in the surrounding area around the nucleus of an atom.
Because electrons are so small, the majority of the mass is made up of solely the nucleus (protons and neutrons).
Symbols
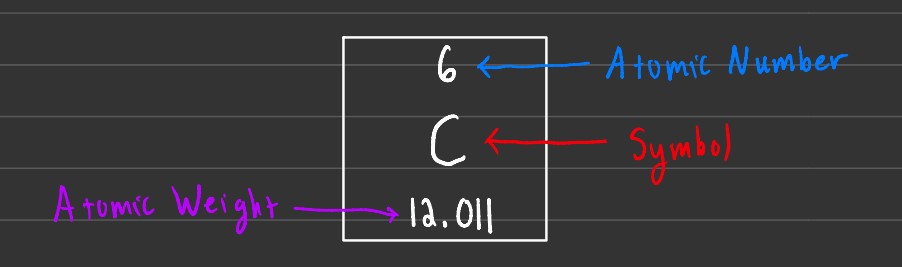
Atomic symbols are used to give us an easy way to determine the number of protons and neutrons of a specific atom. To find the number of protons, you check the atomic number. To find the number of neutrons in an atom, you subtract the atomic number from the atomic weight.
Atomic weight is measured in amu or atomic mass units. 1 amu is approximately 1.6606 x 10-27 kg. Note that the atomic weight isn't an integer. This is because atomic weight, or in this case 12.011 amu, is the average mass of an atom across all its isotopes.
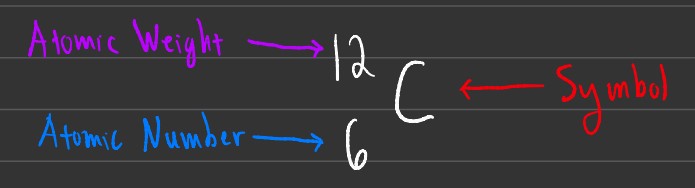
This is another way to write atomic symbols, and essentially has the atomic weight and atomic number flipped. This notation is often used when looking at 2 different isotopes of a specific element.
Isotopes

Isotopes are atoms that have the same number of protons, but a different number of neutrons. In the example above, both atoms are carbon. The main difference is that the right-most carbon atom has an atomic weight of 13 compared to 12 of normal (stable) carbon. This means that this carbon has 1 extra neutron compared to the left carbon.
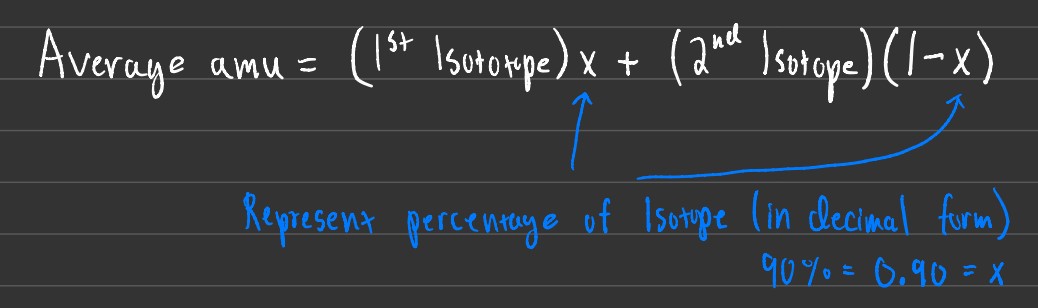
Several problems can be given to you revolving around isotope abundance. Some may want you to find the average amu, while others may want you to find the % of each isotope given an average amu.
Using the formula above you can calculate for either average amu, the percentage of each isotope, or even for the weight of each isotope.