Balancing Chemical Equations
When dealing with chemical equations there are several components, such as reactants and products alongside the state of the compounds. This sub-section aims to help you understand what role each part plays and how to balance chemical equations!
Parts of an Equation
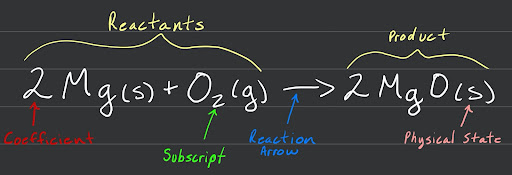
When reading a chemical equation, generally the left side will be the reactants while the right side is the products. The equation will also tell you the physical state of each element, its subscripts, and its coefficients.
Steps to Balance Equations
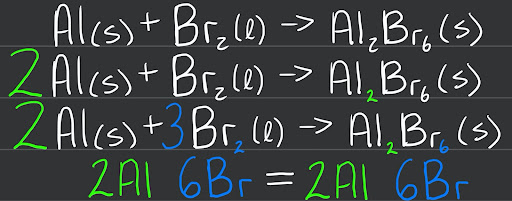
Balancing an equation is a relatively straightforward process, involving the addition of coefficients to either the reactants or products side or both sides in some cases. The steps to balance an equation are as follows -
Write correct formulas for reactants and products (including their physical states).
Balance the compound with the most elements in the formula first using integers as coefficients.
Balance isolated or lone elements last.
Check to see that the sum of each individual element is equal on both sides of the equation.
If coefficients can be simplified by dividing through with a whole number, do so.
Note: It helps to keep 2 lists of each element to keep track of the number of atoms, one for the reactants side and another for the products side. This will help determine the coefficient to use.