Lewis Dot Structure
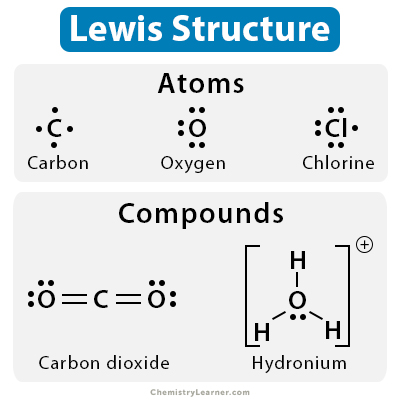
These are representations of molecules and show all the valence electrons, bonds (represented by lines), and nonbonding (represented by dots).
The single lines represent 2 electron bonds, while each dot represents a singular electron.
Octets & Forming Diagram
Generally, each atom has 8 electrons in its valence shell (with the exception of hydrogen). In order to create a Lewis Dot Structure diagram, you need to follow these steps.
Find the sum of all valence electrons in the molecule or polyatomic ion.
- Add 1 electron per charge if it is an anion (negatively charged).
- Remove 1 electron per charge if it is a cation (positively charged).
The central atom should be the least electronegative out of them all (however this should never be hydrogen).
Connect the outer atoms to the central by single bonds (each bond is 2 electrons).
Next, you should fill the octets of the outer atoms, and once these are all filled you should move on to the central atom and fill its octet (outer shell).
If you do not have enough electrons to fill the octet of the central atom, form multiple bonds and then try filling the central octet again.
Octet Rule Exceptions
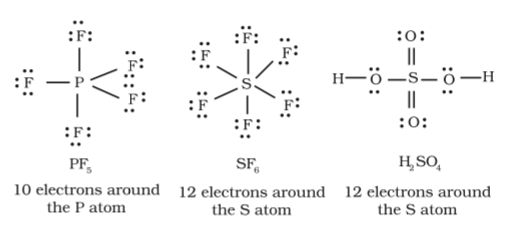
There are some cases where the octet rule does not apply. Usually, this occurs at and beyond the 3rd row of the Periodic Table. These atoms can have expanded octets, meaning that they have more than 8 valence electrons in their outer shell.
Boron and beryllium are also octet exceptions, with them generally not gaining a full octet by sharing electrons.
Note: The best Lewis Dot Structure is the one that minimizes formal charge. This can be seen in the next section where formal charge is talked about.