Types of Equations
There are 3 main forms of chemical equations. The
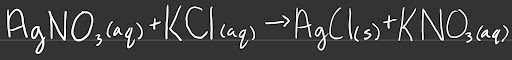
Molecular equations show all substances as molecular formulas, keeping compounds/molecules together and displaying the physical states of the molecules.
Total Ionic equations show all ions and molecules, including spectator ions. Spectator ions are on both sides of the equation and are non-active participants in the reaction.
In the case above, the spectator ion is NO3- (aq)
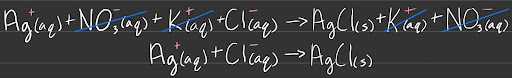
Net Ionic equations show only the ions and molecules that participate, so spectator ions are emitted.
In the example above, the spectator ions are NO3- (aq) and K+ (aq) which are ommitted from the Net Ionic Equation.