Heat Capacity
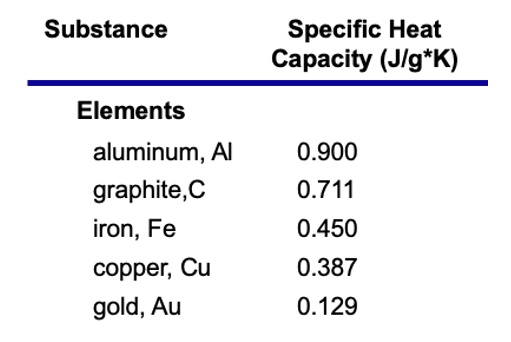
This property, heat capacity, is largely important in thermodynamics and in both this and the next chapter.
Specific & Molar Heat Capacity
When referring to the specific heat capacity of a substance or an object, it means the amount of heat (q) required to raise the temperature of 1 gram of the substance/object by 1 degree Celsius. This is shown by an uppercase C.
- An example can be shown by water (H2O), which has a specific heat capacity of approximately 4.184 J/g°C.
- An interesting trend shows that metals have a lower specific heat, meaning that it's easier to change the temperature of a metal.
Molar heat capacity on the other hand (represented by Cm) is similar to specific heat capacity. The main difference is that this is the amount of heat required to raise the temperature of 1 mole of a substance by 1 degree Celsius.
Note: Temperature is different from heat.
First Law of Thermodynamics
Energy can be transferred from one form to another, but it cannot be created or destroyed. The total energy in the universe is constant.