Heat Transfer & Calorimetry
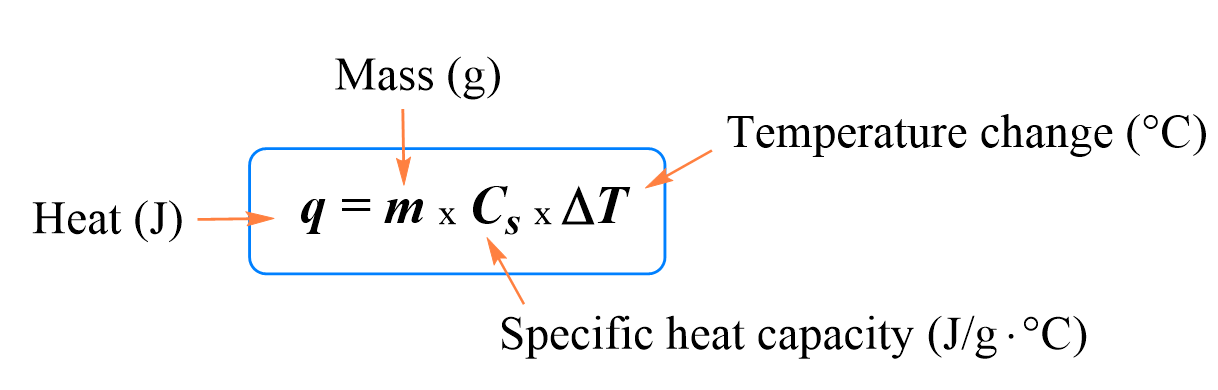
The formula shown above is one of the most common you will see throughout this chapter, it connects heat, mass, specific heat, and temperature change.
Heat Transfer
When it comes to heat transfer, specific heat plays a large role in the amount of heat/energy required to change the temperature of a substance/object.
High-specific heat
- Takes lots of heat to change the temperature by 1°C.
Low specific heat
- Takes little heat to change the temperature by 1°C
The equation shown above is used fairly often, and it's important to note that the qsystem = - qsurroundings and vice versa.
Calorimetry
Calorimetry itself is an experimental technique used to measure the heat flow into or out of a thermodynamic system. What this means is it basically measures the change in heat.
Another important thing to note is that qreaction = - qcalorimeter. There are 2 main kinds of Calorimetry, coffee cups, and bomb calorimetry.
Coffee cup calorimetry
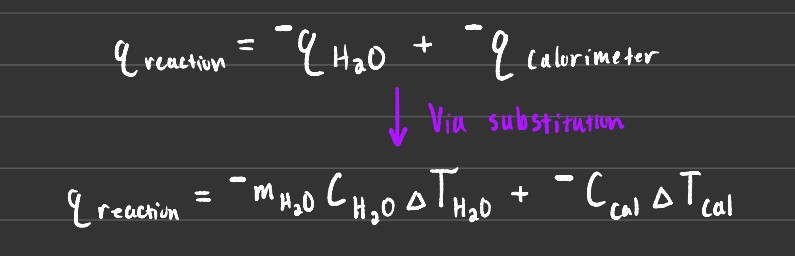
These types of calorimetry have their independent formulas and vary slightly as shown above for the coffee cup.
Bomb Calorimetry
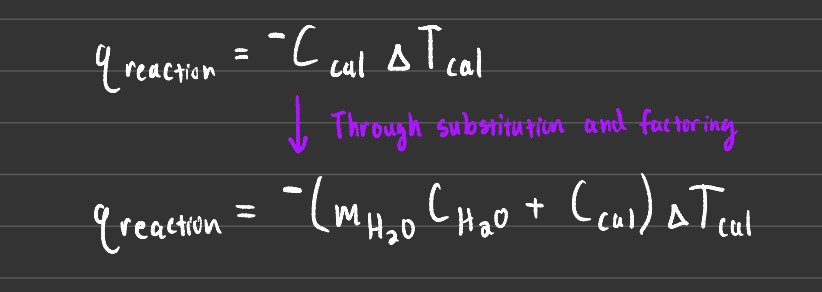
The above image shows the different formulas that can be used when dealing with bomb calorimeters.