Bonds
Earlier in the year, different types of chemical bonds were talked about. These included ionic, covalent, and metallic bonds. Within covalent bonds, there are different naming conventions used. The most common are sigma and pi, used to term the number of covalent bonds between 2 atoms.
Sigma bonds are denoted by the σ symbol. Every single bond formed is a sigma bond.
Pi bonds on the other hand are shown by the π symbol. Double bonds are 1 sigma and 1 pi bonds, while triple bonds are 1 sigma and 2 pi bonds. You will always have a sigma bond first, and any subsequent bonds will be pi bonds within the same connection.
Strength & Length
Bond strength and length are directly related to the number of electrons shared between 2 atoms. The more atoms are shared, the stronger and shorter the bond will be.
Generally, Triple bonds > Double bonds > Single bonds, when it comes to the strength of the bond. The opposite is true when it comes to length, with Single Bonds being the longest while Triple bonds are the shortest.
Dipole & Polar Bonds
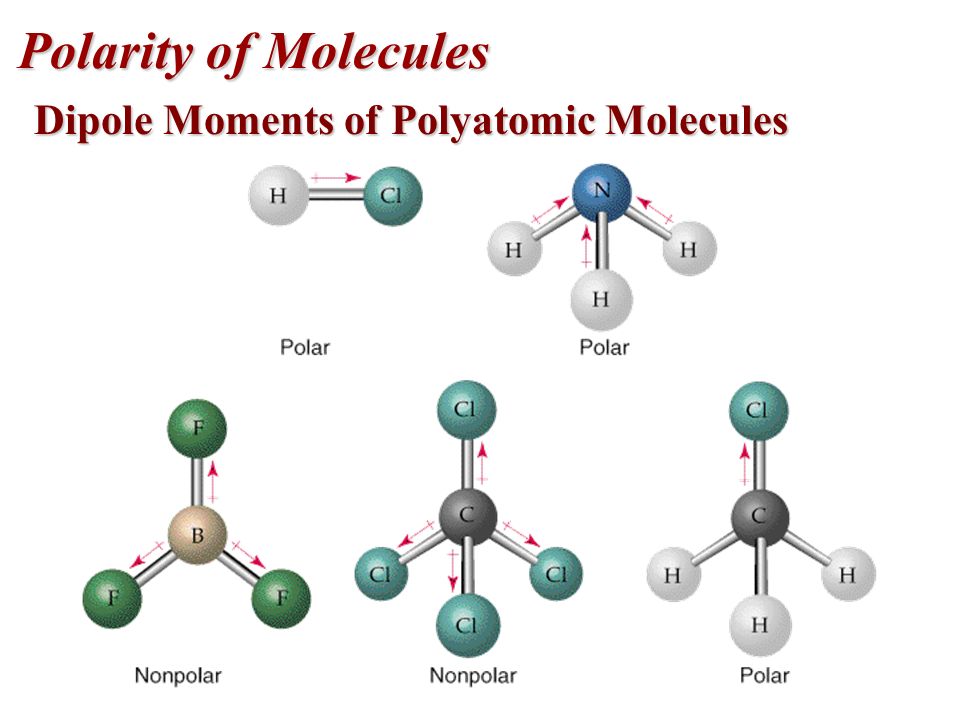
Molecules can be either non-polar or polar, and when they're polar they have a dipole moment. Dipole moments create a positive and negative side of a molecule, with the molecule's atoms and electrons shifting accordingly.
2 atoms can be polar, but the overall molecule can still be non-polar. This is shown in the bottom left example, where fluorine is more negative while boron is more positive. Because this polar dipole moment occurs on all sides, it cancels out and as a result, the molecule is non-polar.