Gibbs Free Energy
Gibbs Free Energy (∆G) is the energy that is available to do useful work in a spontaneous process (∆G < 0) or the work that is required to drive a non-spontaneous (∆G > 0) process. Heat energy cannot be completely transferred into work energy due to entropy.
- The formula is: ∆G = ∆H - T∆S
For example:
When a reaction is exothermic and the disorder increases, the reaction is -
- Exothermic means ∆H° < 0.
- Disorder increases means ∆S° > 0.
- Looking at the equation ∆G° = ∆H° - T∆S°, we can conclude that ∆G° < 0 for all values of T. Therefore, the reaction is spontaneous at all temperatures.
When a reaction is exothermic and the disorder decreases, the reaction is -
- Exothermic means ∆H° < 0.
- Disorder decreases means ∆S° < 0.
- Looking at the equation ∆G° = ∆H° - T∆S°, we can conclude that ∆G° < 0 for small values of T. Therefore, the reaction is spontaneous at low temperatures and not spontaneous at high temperatures.
Calculating Gibbs Free Energy (ΔG)
The formula to calculate Gibbs Free Energy is:
- ∆G°rxn = 𝚺 n ∆G°f(products) - 𝚺 m ∆G°f(reactants)
N and m are the stoichiometric coefficients of each product or reactant.
Similar to ∆S, If a reaction is carried out in a series of steps, ∆G for the overall reaction will be equal to the sum of the ∆G’s for the individual steps.
Crossover Temperature
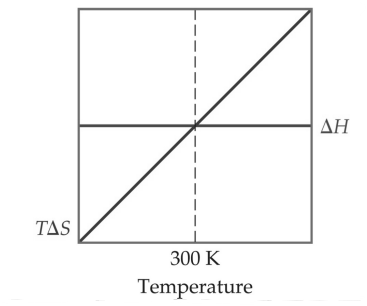
When the equation ∆G° = ∆H° - T∆S° is at equilibrium, ∆G = 0. This point is the crossover of two lines as shown in the diagram below. The temperature at this crossover is usually the boiling point or melting point.