Diffusion and Effusion
Diffusion and Effusion are two very similar but different concepts. When several different gases mix by random molecular motion and collision, they are diffusing. However, when gas molecules escape without collision through a tiny hole in a vacuum, they are effusing. The image above serves as a visual representation of diffusion vs. effusion.
Rates of Diffusion and Effusion
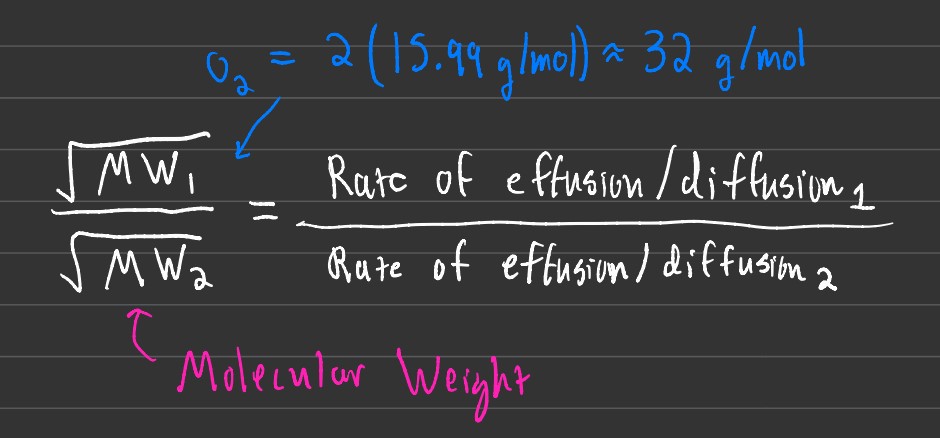
The rate of diffusion is used to find out how quickly gas is being diffused amongst other gases or mixing. Effusion rates, however, are used to determine the rate of gas escape.
Density will inversely affect the rate of diffusion/effusion. The denser the gas, the slower it'll be able to diffuse or effuse.
Important: When attempting to find the rate of diffusion or effusion, put the element you want to find the rate of at the bottom of the equation!
