Kinetic Molecular Theory
The Kinetic Molecular Theory and its statements are only true if a gas behaves ideally, and the other way around. This theory explains why gases behave the way they do, and how volume, pressure, temperature, and moles of a gas are all related.
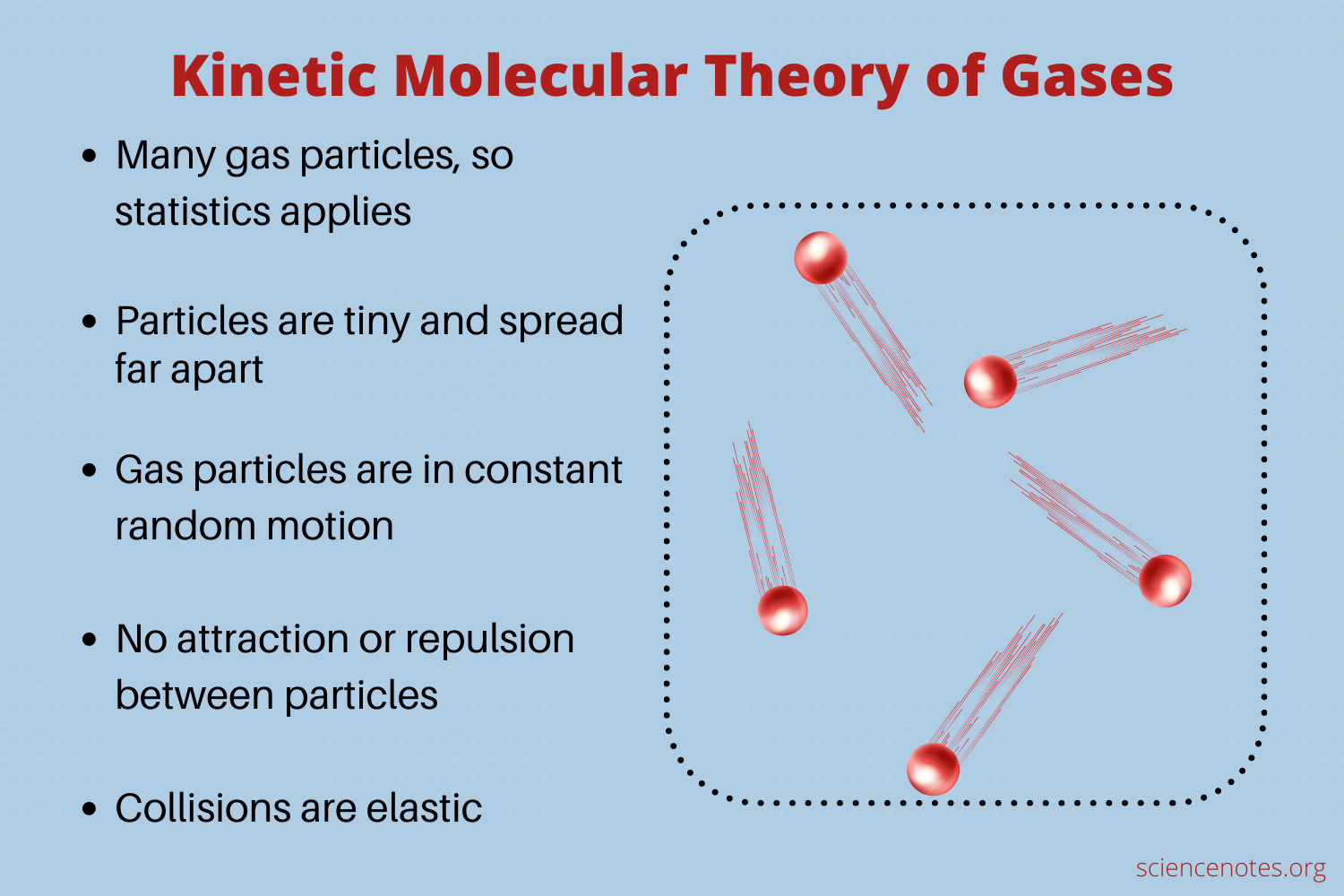
Gas is made up of many particles which are in constant, random, motion.
Particles are infinitely small, therefore they have no volume.
Particles move in straight lines unless they collide with other molecules or the walls of the container.
Collisions are elastic, so the total kinetic energy of the particles is conserved.
- KE = 1/2 (mv)2
Particles in a gas interact with each other only when collisions occur.
The average kinetic energy of the particles in a gas is proportional to the absolute temperature of the gas and does not depend on the identity of the gas itself.
Note: Not all gases behave ideally, specifically those that have a low temperature and high pressure.