Resonance & Formal Charge
There may be multiple Lewis Dot Structures for a molecule or polyatomic ion, so how do you choose the best one? This is where resonance and formal charge come into play.
Resonance
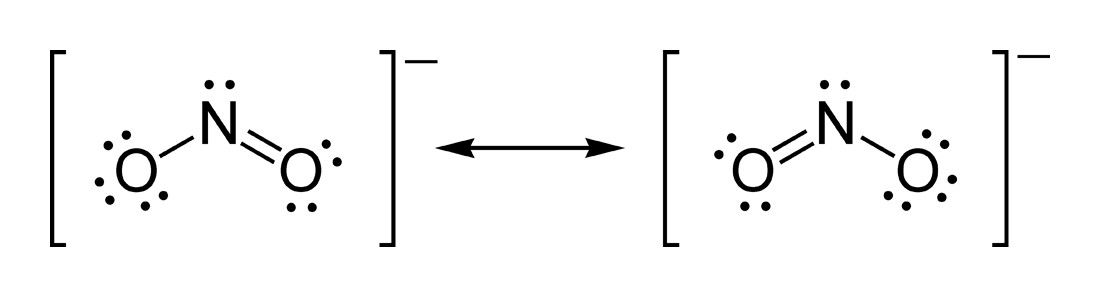
In some cases, there will be multiple ways to draw a Lewis Dot Structure for a molecule or polyatomic ion.
When this happens, the molecule goes between the possible structures and is both at the same time. The bond lengths in the resonance structures are equal.
Formal Charge
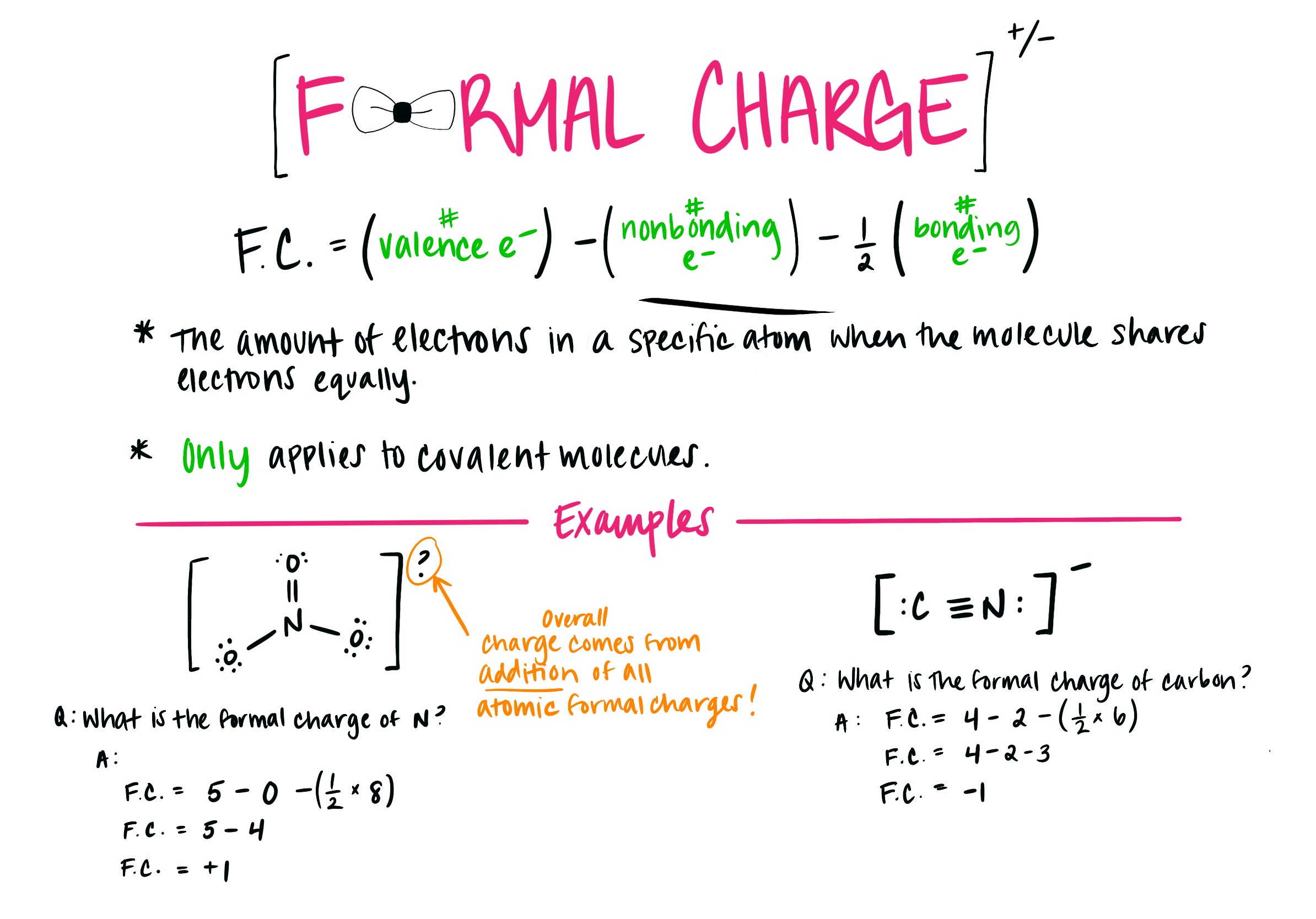
Formal charges are most often used to find the best Lewis Dot Structure, which is always the one that has the lowest formal charge.
Formal charge itself is found by taking the valence electrons and subtracting them by the lone electrons, then subtracting the number of bonds. The result will be your formal charge, with the sum of all formal charges being the ending charge on the molecule as shown above.