Molecular Geometries
The shape of a molecule is determined by several things, with one of the most important being the electron domain. This combined with the atoms and lone pair electrons gives you the Molecular Geometry.
VSEPR
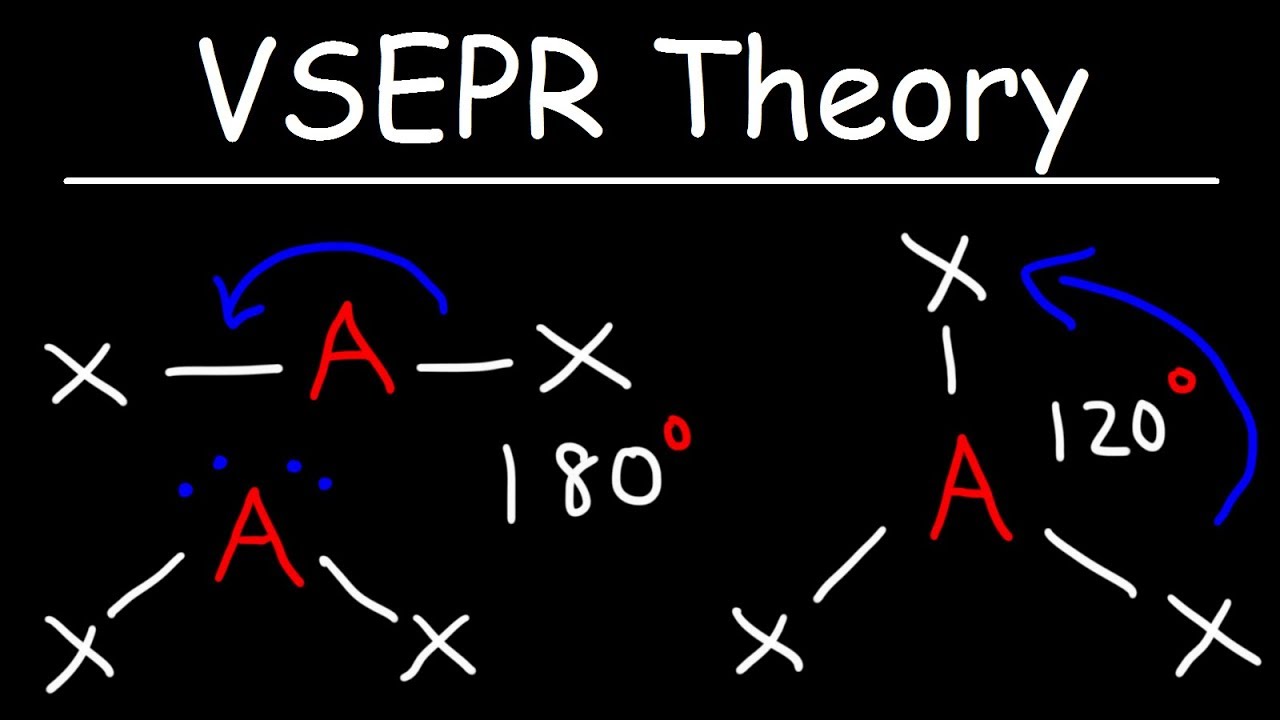
This acronym stands for "Valence Shell Electron Repulsion Theory", which essentially says that electron pairs will try and push each other so they are placed as far apart as they can to minimize this repulsion.
Additional information on this theory can be found here.
Electron Domains
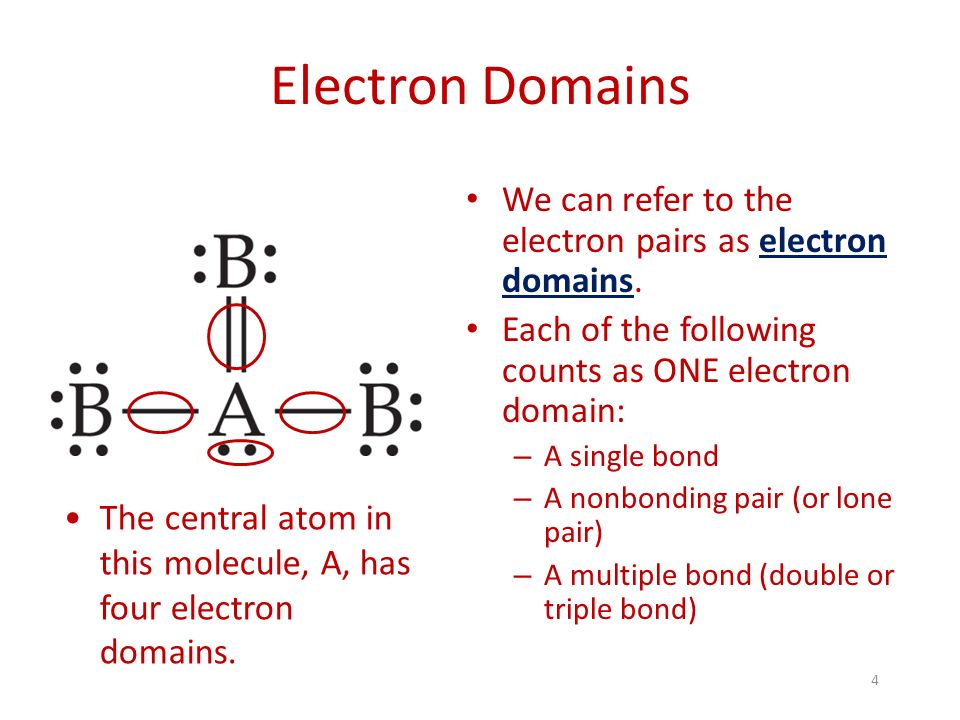
To determine the Electron Domain of a molecule or polyatomic ion, look at the central atom and find the number of lone pairs of electrons plus the number of atoms bonded to the central atom.
The Electron Domain Geometry is often not the shape of the molecule, this is usually found in Molecular Geometry by looking at the types of bonds and locations of lone pairs of electrons.
Molecular Geometry
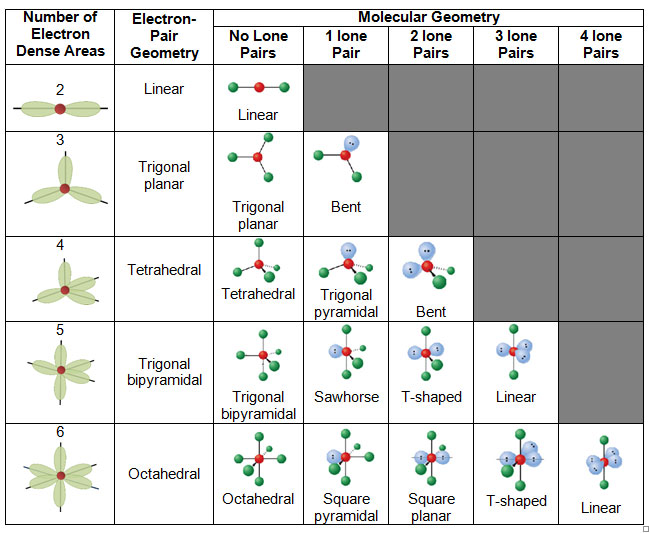
The molecular geometry of a molecule or polyatomic ion is defined by the positions of only the atoms, not the nonbonding lone electron pairs.
There are different bond angles for each geometric shape, such as follows.
- Linear - 180°
- Trigonal planar - 120°
- Tetrahedral - 109.5°
- Trigonal Bipyramidal - 120° and 90°
- Octahedral - 90° and 180°
Note: These bond angles can be affected if there are double or triple bonds, as the electron density changes.