Entropy
Entropy can be described as the “disorder” of a substance.
So how do you determine which substance has a higher entropy? The 5 main factors that determine the entropy of a substance are temperature, volume, state, number of particles, and the size/complexity of a molecule.
In most cases, to determine the entropy you should -
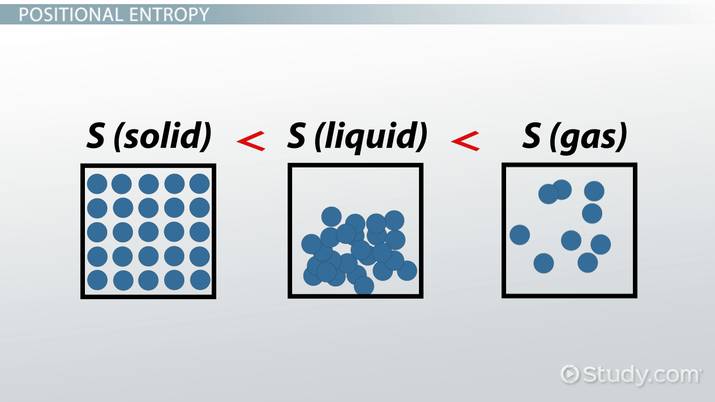
First check the state:
- Gas has the highest entropy, followed by liquids, and lastly solids.
Check the amount:
- The substance with the largest number of moles generally has the highest entropy.
By the elements:
- The compound with the most elements has the most entropy.
Examples -
H2O (l) vs H2O (g)
- H2O (g) has the highest entropy because it is a gas which beats liquid.
HCl (g) vs Ar (g)
- HCl (g) has the highest entropy because it is composed of two elements compared to Ar which is only one element.
We can use this skill to find out the sign of ΔS of any reaction.
More examples -
Ag+ (aq) + Cl- (aq) → AgCl (s)
- The product has a lower entropy because it is a solid. Therefore, ΔS < 0.
4Fe (s) + 3O2 (g) → 2Fe2O3(s)
- The reactant has a higher entropy because there is a gas, and there are more mol’s. Therefore, ΔS < 0.
Raising the temperature of 100g Cu from 275 K to 295 K
- The product has a higher entropy because when heating a substance you make the particles move faster which is like creating a disorder.
Note: There is actually a way to calculate entropy, which is shown in the next section.