Standard Entropy of Reaction
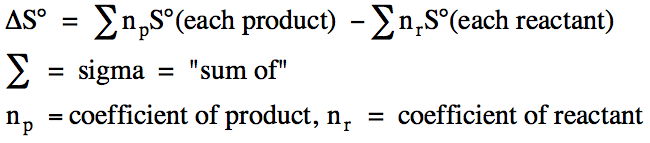
Where n and m are the stoichiometric coefficients of each product or reactant.
If a reaction is carried out in a series of steps, ∆S for the overall reaction will be equal to the sum of the ∆S’s for the individual steps.
For example, we want to determine the change in entropy for the following reaction.
- A + 2B → C + 2D
The steps needed to achieve this reaction are as follows:
- 2E + A → C
- ∆S1
- B → D + E
- ∆S2
In order to get the overall reaction, we would need to do step 2 twice. This will lead to an overall change in entropy of ∆S = ∆S1 + 2∆S2.
Calculating Entropy
The ∆Ssys, alone, is not an indicator of spontaneity. However, the ∆S of the universe (system + surroundings) is enough to determine spontaneity.
The 2nd Law of Thermodynamics states that the entropy of the universe is increasing during a spontaneous process. In other words:
- ∆S of universe > 0 means spontaneous.
- ∆S of universe < 0 means non-spontaneous.
Exothermic processes increase the entropy of the surroundings (∆Ssurr > 0).
Endothermic processes decrease the entropy of the surroundings (∆Ssurr < 0).
Calculating ∆Ssurr (entropy of surroundings)
The heat that flows into or out of the system changes the entropy of the surroundings. For an isothermal process (const temperature):
- ∆Ssurr = - ( qsys ) / T
At constant pressure, qsys is simply ∆H° for the system:
- ∆Ssurr = - ( Hsys ) / T