Ions
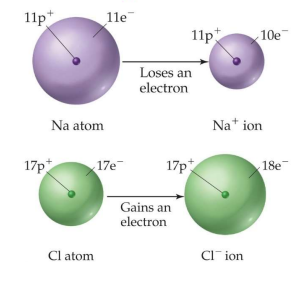
There are several types of ions, and most are formed when atoms lose or gain electrons. The main 2 types of ions are monatomic ions and polyatomic ions. Ions are then classified by whether they have a positive charge or negative charge, leaving them classified as either a cation or anion.
Classifying Ions
Monatomic ions are charged particles that form when a single atom gains or loses one or more electrons. An example would be Na+, Cl-, Al3+ etc.
Polyatomic ions on the other hand are a group of atoms that carry an electrical charge. Examples include ClO3-, CN- etc.
Beyond being classified as monatomic or polyatomic, an ion is also referred to as either a cation or anion.
Cations (+)are positively charged monatomic or polyatomic ions, which have more protons than electrons.- Add -ion to the end of monatomic cations.
- Ex. Sodium -> Sodium Ion, Na+
Anions (-)on the other hand, are negatively charged monatomic or polyatomic ions. This means that anions have a negative charge and more electrons than protons.- Add -ide to end of monatomic anions.
- Ex. Chlorine -> Chloride, Cl-