Compounds and Chemical Bonds
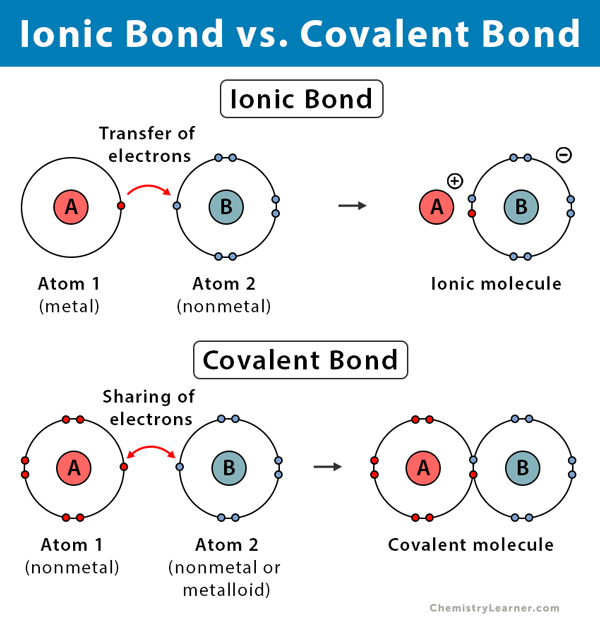
Compounds are formed when 2 atoms of different elements bond. These bonds may occur due to atoms wanting to fill their valence shell with electrons, or give away electrons to have an octet. This is because having a full octet (full valence shell) makes atom stable.
Ionic Bonds
- These bonds form between non-metals and metals, where the anion (negatively charged atom) transfers its extra electrons to the cation (positively charged atom) and forms an ionic compound.
- Ex. NaCl
Covalent Bonds
- These bonds form between 2 non-metals and involves the two atoms sharing their electrons to satisfy both valence shells and become stable.
- Ex. H2O
Metallic Bonds
- In metallic bonds electrons create a valence electron sea, allowing electricity to easily be conducted.
- This isn't much of a bond, it's more like a sea of floating electrons that are all loosely connected to one another.