Molecular vs. Empirical
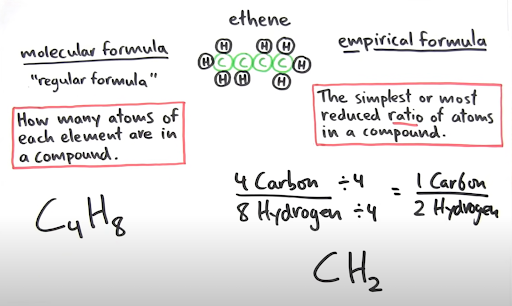
There are 2 general representations of a formula, those being molecular and empirical. The molecular formula is the regular/actual formula for a compound or molecule, showing how many atoms of each element are in the compound. An example of this is shown above, as C4H8. This formula is essentially a multiple of the empirical formula.
On the other hand, the empirical formula is the simplest ratio of atoms in a compound. To find the empirical formula, divide all of the subscripts in the molecular formula by a common denominator. The result will give be the empirical formula.
Mass Percentages

Finding the mass percentage of an element is a relatively straightforward process.
First, identify the element you want the mass percentage of.
Find the total number of atoms within the compound (found by inspecting coefficients and subscripts).
Then multiply this number by the atomic weight of the element, or amu.
Once you have this result, divide by the full weight of the compound and multiply by 100 to find the mass percentage of the element.