Periodic Trends
Although atoms come in all different sizes and have many different properties, there are general periodic trends that can be used to identify their size and/or energies associated. Here are 4 of the most important periodic trends in CHEM 107.
Atomic Radii
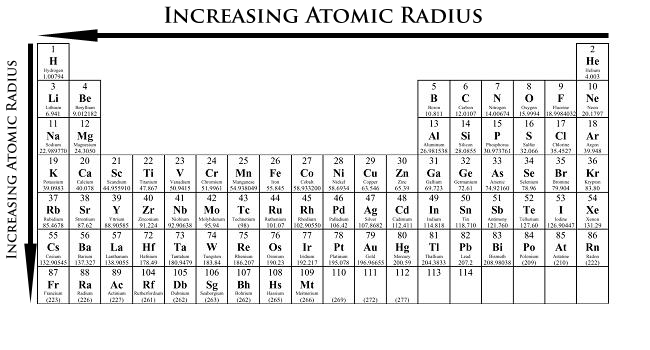
The size of an atom is largely dictated by the number of protons and electrons it contains. Atomic Radii increases when moving from right to left across the periodic table, and moving from top to bottom.
The reason that atomic radii (or the size of an atom) decrease going from left to right is that for each proton added, one electron is also added. In theory, this should make the atom bigger, right? No, not exactly. Having more protons and electrons in the same shell or period (row) means a greater nuclear charge or pull towards the center. This makes the atom denser as it moves from left to right across the periodic table.
Ionization Energy
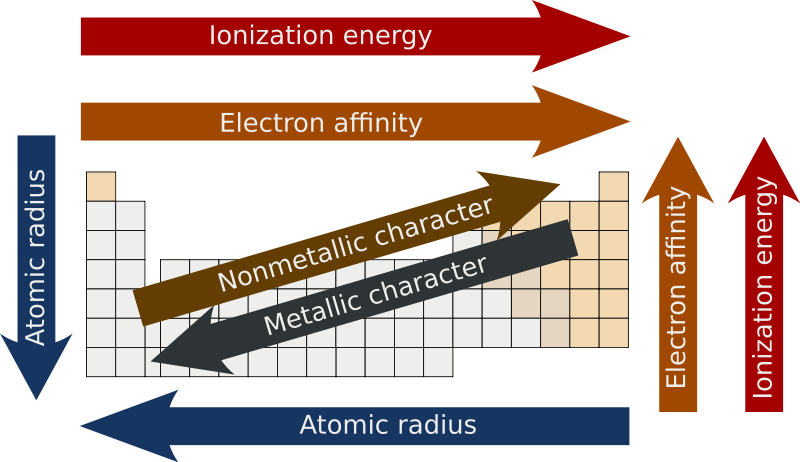
Note: Although ionization energy and electron affinity both have the same periodic trend, they are 2 very different things.
gnore the nonmetallic and metallic characters, they are not relevant when it comes to periodic trends.
Ionization energy refers to the amount of energy required to remove an electron from an atom. As shown above, the required energy increases when moving from left to right in the periodic table. This trend is due to the number of electrons within an atom increasing across the period (row), and is especially noted when it comes to noble gases.
Because noble gases have a full octet (8 electrons) in their outermost shell (except for helium), they are very non-reactive and do not want to give up their electrons. Because of this, it takes a very large amount of energy to remove an electron from its outer shells.
Electron Affinity
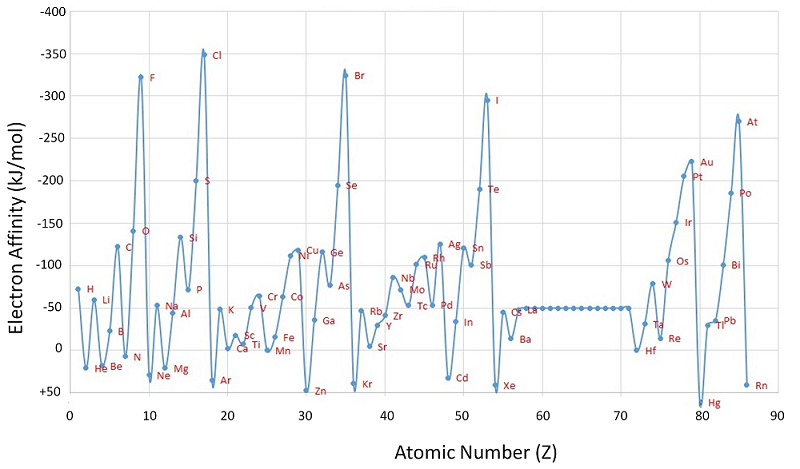
Electron affinity on the other hand, represents the change in energy that comes with adding an electron to an atom. This energy change is negative when an atom wants the electron, so when it is added energy is released (hence the negative symbol). However, when measuring the electron affinity of atoms such as neon (a noble gas), the electron affinity is positive. Why is this? Well, the neon atom is happy with its full octet, so it doesn't want to accept another electron. The energy "released" is then positive because it will take extra energy to force neon to accept the extra electron.
Electronegativity
Electronegativity measures the tendency of atoms to attract electrons, so a high value means that the atom strongly attracts shared electrons. Electronegativity has the same periodic trend as both ionization energy and electron affinity but is different and independent of both.
An example of a very electronegative element or atom is fluorine, with a value of ~ 3.98. Fluorine is one of, if not the most electronegative element on the periodic table. This is because the outer shell is missing only 1 electron, so the atom desperately wants 1 more electron to fill its octet and become stable.
Generally, as you go up and to the right of the periodic table, elements become more electronegative. There are exceptions, however, such as noble gases. This is because they are already stable, and do not want any more or fewer electrons than what they already have. Therefore, they don't react nor have an electronegative value.