Electrolytes
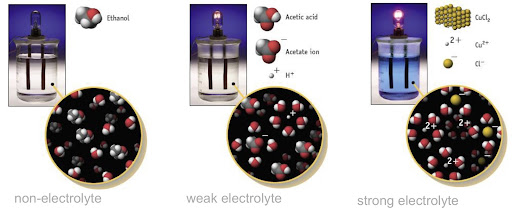
Electrolytes are substances that, when dissolved in water, conduct electricity. Non-electrolytes are substances that don't conduct electricity when dissolved in water.
Strong and Weak Electrolytes
There are 2 kinds of electrolytes, strong and weak. Strong electrolytes exist in solutions and tend to completely dissociate and break down into ions. The following are strong electrolytes -
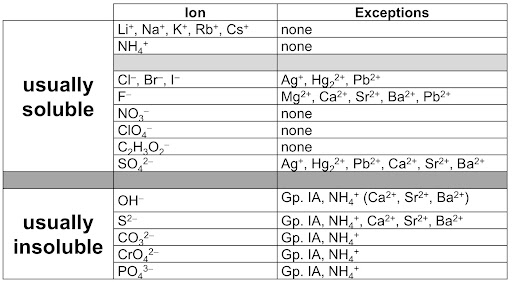
- All strong soluble salts (shown above)
- All strong acids (HCl, HBr, HI, HNO3, H2SO4,HClO4)
- All strong bases (NaOH, LiOH, KOH, ~Ba(OH)2)
Weak electrolytes on the other hand are solutes that exist in solutions mostly in the form of molecules (they don't completely dissociate into ions).