Solubility
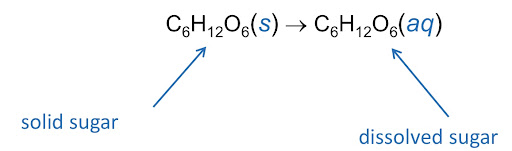
The above image shows a solid (s) becoming an aqueous solution (aq) by dissolving in water. Aqueous solutions will be talked about in more detail in the following sub-section.
Solutions
A solution is a homogenous mixture of two or more substances and can be solid, liquid, or gaseous. There are 2 parts within a solution, the solvent, and the solute.
- A solvent is the substance present in the greatest quantity, while a solute is a substance other than the solvent.
- Solutes are said to be dissolved in the solvent.
Essentially, a solvent is what the solute dissolves in (so if sugar is dissolved in water, the solvent would be water, and the solute would be sugar). That would make the resulting solution aqueous (aq), which is a liquid solution in which the solvent is water.
When there's too much solute for the amount of solvent, a solution becomes saturated and precipitates form (a solid that falls out of solution).
Check out Electrolytes Page for a chart showing what ions are soluble and under what conditions.
Soluble Compounds
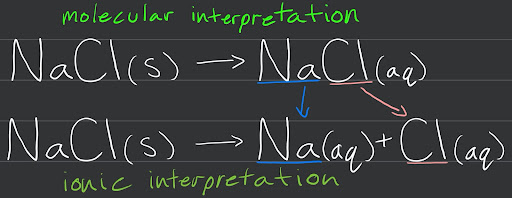
There are 2 kinds of soluble compounds, molecular and ionic. The image above shows the difference between soluble molecular and ionic compounds, and how they act whenever they become aqueous.
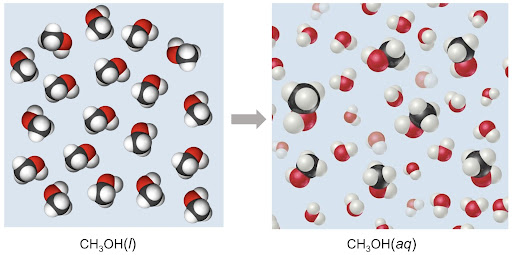
Molecular compounds generally remain intact within the solution and are surrounded by polar water molecules. An example of that is shown above, as the compound/molecule itself stays together within the solution.
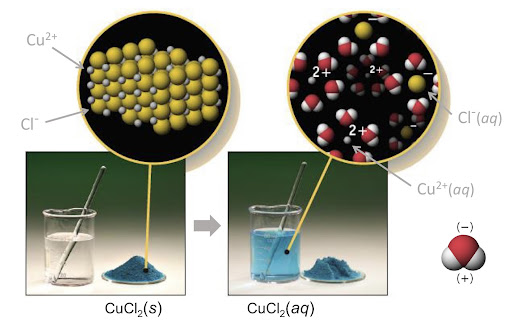
Above is an example of an ionic solution. Soluble ionic solids (also known as salts) tend to break up or dissociate to form ions within an aqueous solution (aq). These ions are then surrounded by polar molecules such as water.