Bohr Model and Electronic Structure
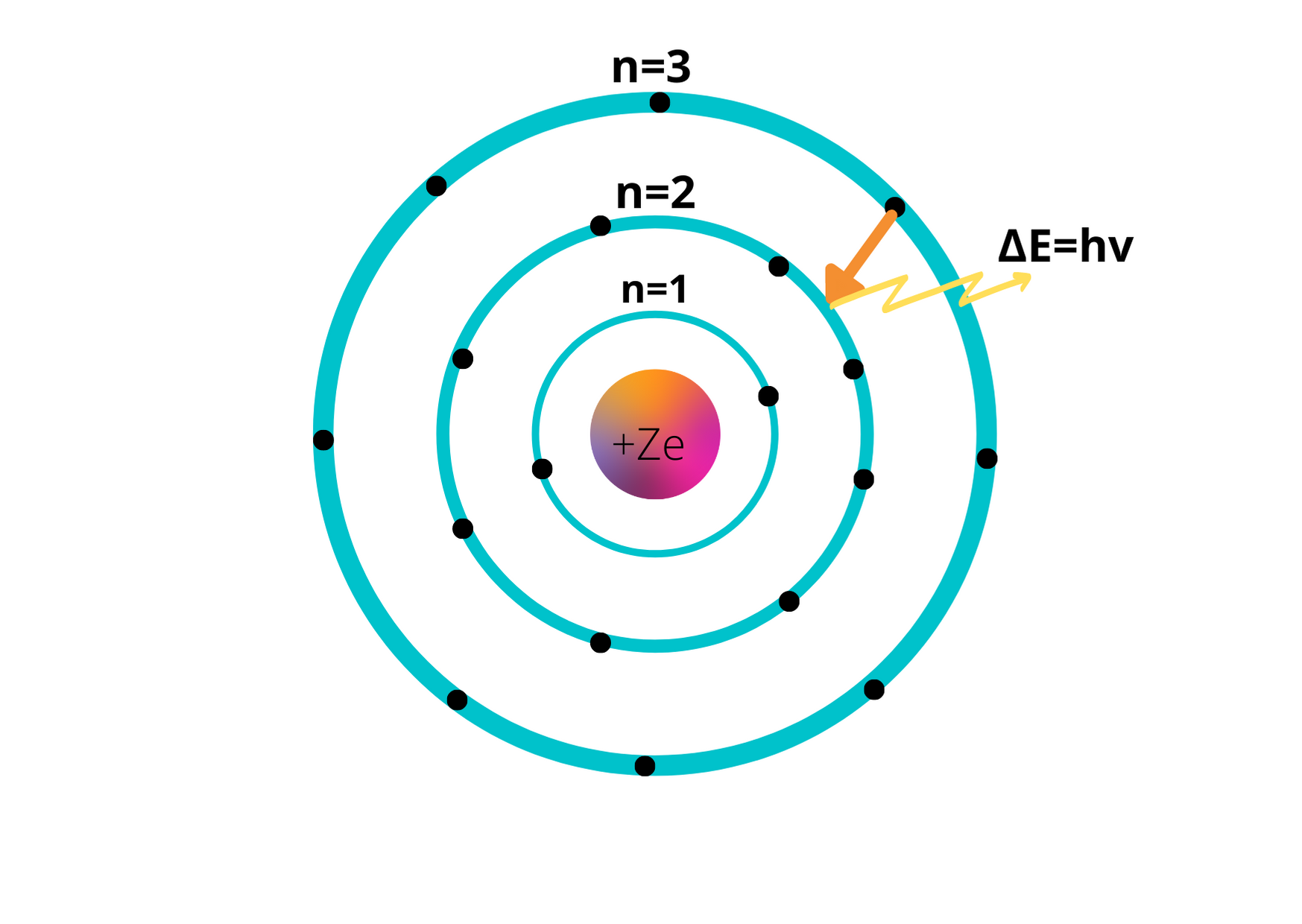
In the Bohr Model, electrons are depicted as orbiting the nucleus in levels. Additionally, there are two distinct states that can exist within each atom or molecule.
Energy Transitions and States
The two states that exist within the Bohr model of the atom are the ground state and the excited state. The ground state refers to the lowest energy state of an atom, while the excited state refers to an atom absorbing energy and its electrons being promoted to a higher energy orbital.
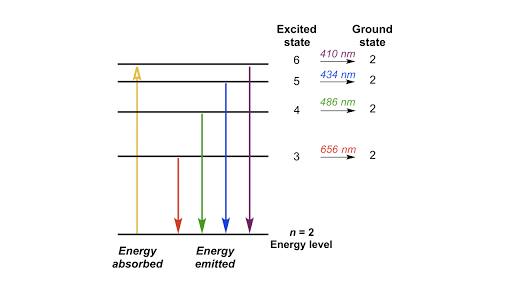
The process of an atom obtaining energy and promoting electrons to outer orbitals is called absorption, where the atom aquires or "absorbs" photons. For example, when an atom gains energy through photons, its electrons may jump from the n = 1 orbital up to the n = 2 orbital. This excited state, however, cannot exist forever due to the electrons losing that energy and transitioning back down to ground state through a process known as emission. In emission, an atom releases or "emits" photons or energy and transitions back down to the original ground state.
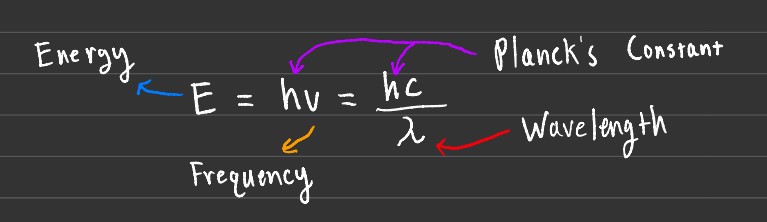
The emission and/or absorption wavelengths can be calculated using the formulas shown above, alongside the energy it may take to transition from different orbital levels.