Energy
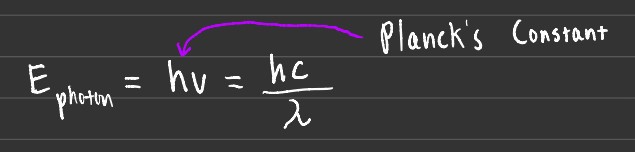
Energy, frequency, and wavelength are all related. This statement holds true in this sub-section and many if not all that follow. Energy is generally measured in joules. One important constant that will be used is Planck's Constant. This constant is denoted by h and is approximately 6.626 x 10-34 Js.
Photoelectric Effect
Light is very unique, being that it expresses properties of both a particle and wave, noted during the photoelectric effect. This was first discovered when electrons were emitted from metal due to light of a specific frequency shining on it.
In order for a particle to be ejected from its system due to the photoelectric effect, the energy that the photon imparts on the particle must be greater than the energy that binds it. These photon's are essentially just small packs of energy, whose energy can be calculated using the formula above.