de Broglie Wavelength & Magnetism
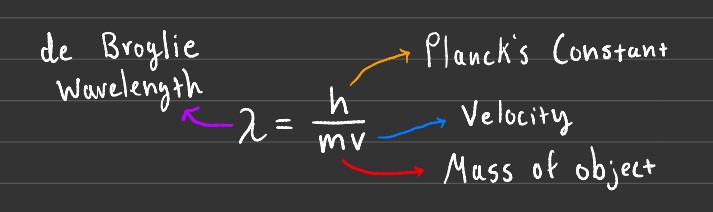
The de Broglie Wavelength and mass of an object are inversely proportional, as the mass of the object increases its corresponding wavelength decreases.
Heisenburg Uncertainty Principle
This principle states that it is impossible to both know the position and momentum of a particle at the same time with reasonable accuracy. The uncertainty in position for lighter objects is relatively large, while the uncertainty in position for heavier objects is relatively small.
Magnetism
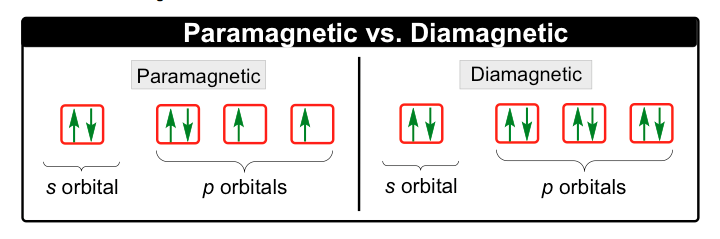
There are two types of substances, diamagnetic and paramagnetic. The main difference between these two is that paramagnetic substances are influenced and attracted to magnetic fields, while diamagnetic substances are repelled by magnetic fields. Diamagnetic substances are repelled because they have no unpaired electrons.
Paramagnetic substances on the other hand, are attracted to magnetic fields due to the unpaired electron they have. This is associated with spin which will be talked about it in more detail in the next sub-sections along with orbitals.