Quantum Numbers & Atomic Orbitals
Over many years, flaws were found within the Bohr model. Schrodinger then came up with another model which instead of having distinct levels, contained a density map with probabilities of where electrons could be at a certain point in time.
Quantum Numbers
Orbitals are what describe the specific distribution of electron density in space. There are 4 primary quantum numbers that describe orbitals. These include the following.
Principal Quantum Number
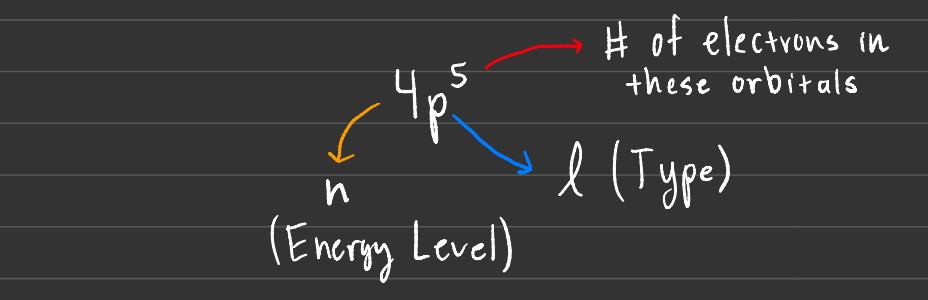
- Denoted by n.
- Value can be 1, 2, 3, or 4.
- Determines shell and the average distance from the nucleus.
- Defines the energy level of the electron in the orbital.
Angular Momentum Quantum Number
- Denoted by l.
- Value can be 0, 1, 2, or 3.
- 0 -> s = "sharp", has a spherical shape with just 1 orbital.
- 1 -> p = "principal", has a dumbell shape with 3 orbitals.
- 2 -> d = "diffuse", has a cloverleaf shape with 5 orbitals.
- 3 -> f = "fundamental", has no definite shape but has 7 orbitals.
- This defines the shape of orbitals.
Magnetic Quantum Number
- Denoted by ml.
- Value ranges from -l to l.
- If l = 2, then ml could be -2, -1, 0, 1, or 2.
- This defines the orientation of the orbital.
Magnetic Spin Quantum Number
- Denoted by ms.
- The value of spin can only ever be +1/2 or -1/2.
- +1/2 indicates spin up.
- -1/2 indicates spin down.
Atomic Orbitals
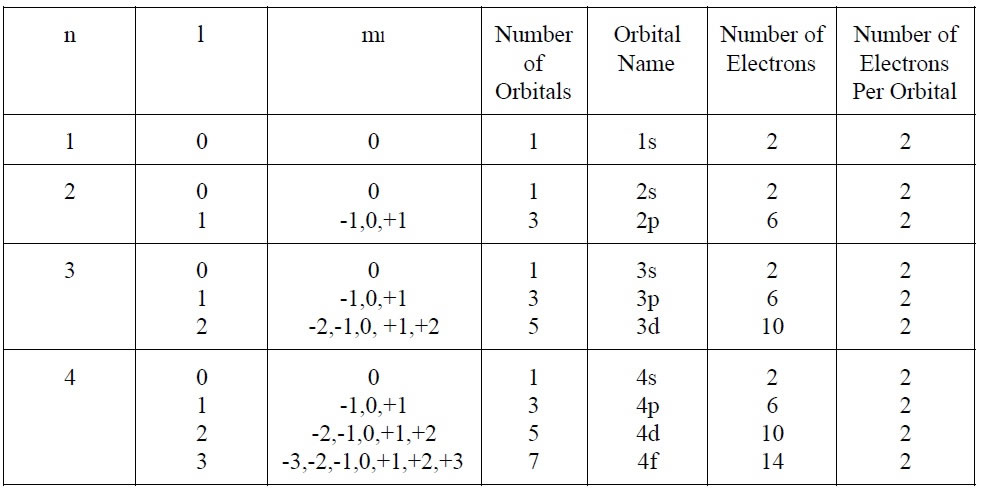
The diagram above serves as a visual representation of Quantum Numbers and shows the relevance of atomic orbitals.
Electrons reside within these atomic orbitals, with up to 2 electrons fitting in each orbital. When an orbital is double occupied or "full", the electrons within the orbitals must have opposite spins.
The total number of orbitals in an electron shell is equal to n2, while the number of orbitals in a given subshell is equivalent to (2l + 1).
Note - The maximum number of electrons is equal to 2n2.