Electrons and Rules
Although there are many atomic orbitals and electrons could simply fill them up without regard for order or structure, there are methods and several rules accompanying the distribution of electrons.
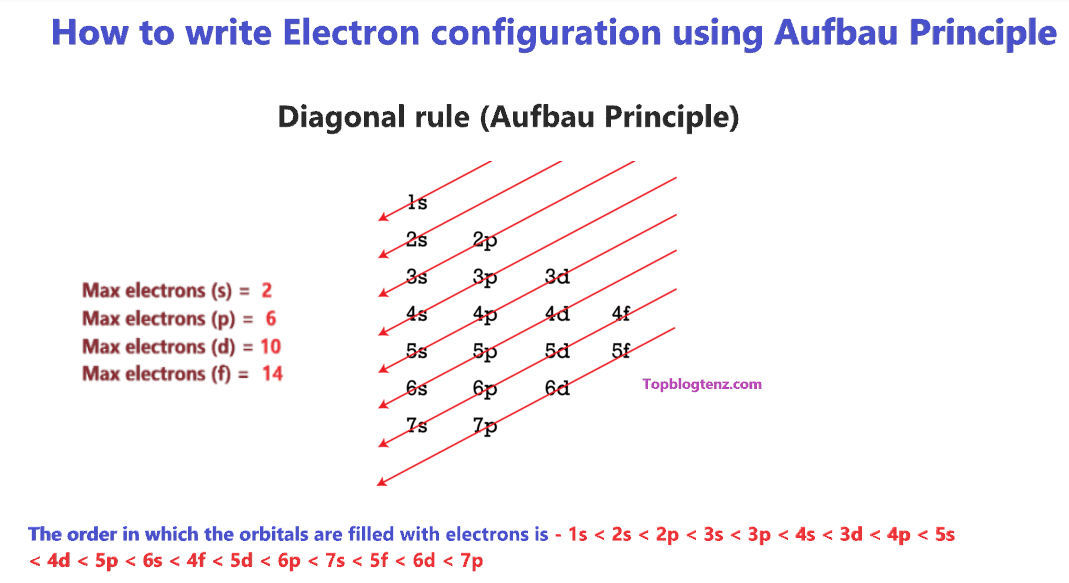
This diagram utilizes Aufbau's Principle to determine the order in which orbitals and subshells are filled with electrons. The electron configuration of a given element can be found by adding electrons to the lowest energy orbitals and progressing until no more electrons can be added. The image above shows which orbitals are filled first and which follow (also showcasing their increasing energy levels).
Note: There are exceptions to the order in which electrons fill orbitals—these include Cr and Cu which will be talked about more in-depth in the next section.
Principles
Pauli Exclusion Principle
- This principle states that no 2 electrons in the same atom can have the same 4 quantum numbers.
- What this means is that each electron must have a unique position within the orbital and shell, including the n, m, ml, and ms quantum numbers.
- So once all of the orbitals within a shell are filled, including all the possible +1/2 or -1/2 spins, there cannot be more electrons added.
Aufbau Principle
- This principle states that electrons must fill lower energy atomic orbitals before progressing and filling orbitals of higher energy.
- The image above showcases this exact principle, with one notable example occurring when 4s comes before 3d. This is because 4s has lower energy than 3d, so it is filled before.
Hund's Rule
- Hund's rule states that every orbital within a subshell (for example 2p) must be filled with 1 electron before a 2nd electron can occupy the same orbital.
- This means that all orbitals in a subshell (for example 2p) must be occupied and have the same spin until another can be added with an opposite spin.
- Because 2p has 3 orbitals it can contain a maximum of 6 electrons, meaning 3 electrons with down spin and 3 electrons with up spin. What Hund's Rule states are that electrons must fill these first 3 spots with the same spin before occupying the same orbital and spinning the opposite way.
- An image showcasing the 3 orbitals is shown in the previous section under magnetism.
- This shows all 3 orbitals being filled singularly before a 2nd electron is added with opposite spin.
Exceptions
These are the 2 notable exceptions that you may encounter later on which don't follow the general rules of electron configuration and filling of orbitals.
The shorthand version of the electron configuration for Cr (Chromium) is Cr 4s1 3d5.
And the shorthand electron configuration for Cu (Copper) is Cu 4s1 3d10.
Both of these fill the 3d orbital before 4s is completely filled, leaving 4s1 instead of 4s2.